Abstract
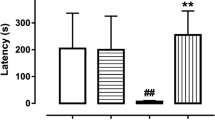
Alzheimer’s disease (AD) is associated with neural oxidative stress and inflammation, and it is assumed to affect more women than men with unknown mechanisms. Kaempferol (KMP) as a potent natural antioxidant has been known to exhibit various biological and pharmacological functions, including antioxidant and anti-inflammatory. We aimed here to evaluate the role of gender difference in response to KMP on the rat model of sporadic AD. Forty-six female and male Wistar rats were divided into six groups of sham, streptozotocin (STZ) + saline (SAL), STZ + KMP. Female rats were ovariectomized, and then all animals received an intracerebroventricular bilateral injection of STZ (3 mg/kg) to induce the AD model. KMP (10 mg/kg) was intraperitoneally administered for 21 consecutive days. Afterward, spatial learning and memory were assessed via the Morris water maze task (MWM). Finally, the hippocampus level of superoxide dismutase (SOD), glutathione, and malondialdehyde were measured using calorimetric kits. Data showed a significant cognition deficit in STZ + SAL compared with the sham. To sum up, we reported that chronic KMP treatment increase significantly improved acquisition and retrieval of spatial memory as evident by longer TTS (total time spent) and short-latency to the platform in MWM. In addition, KMP increased the levels of SOD and glutathione in the hippocampus of rats. Also, KMP decreased hippocampal levels of malondialdehyde in both genders. In conclusion, KMP successfully restores spatial memory impairment independent of gender difference. This memory restoration may at least in part be mediated through boosting the hippocampal level of SOD and glutathione.



Similar content being viewed by others
Data Availability
The datasets and supporting materials generated and/or analyzed during the current study will be available from the corresponding author upon reasonable request.
References
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE (2010) The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE 5(3):e9505
Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B, Long D, Li J (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363
Healy SD, Braham SR, Braithwaite VA (1999) Spatial working memory in rats: no differences between the sexes. Proc R Soc Lond B 266(1435):2303–2308
Rahman Q, Bakare M, Serinsu C (2011) No sex differences in spatial location memory for abstract designs. Brain Cogn 76(1):15–19
Koss WA, Frick KM (2017) Sex differences in hippocampal function. J Neurosci Res 95(1–2):539–562
Yagi S, Galea LA (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44(1):200–213
Chen W, Liu B, Li X, Wang P, Wang B (2020) Sex differences in spatial memory. Neuroscience 443:140–147
Persson J, Herlitz A, Engman J, Morell A, Sjölie D, Wikström J, Söderlund H (2013) Remembering our origin: gender differences in spatial memory are reflected in gender differences in hippocampal lateralization. Behav Brain Res 256:219–228
Klambatsen A, Nygard SK, Chang AJ, Quinones V, Jenab S (2019) Sex differences in memory and intracellular signaling after methamphetamine binge treatment. Brain Res 1711:16–22
Biasibetti R, dos Santos JPA, Rodrigues L, Wartchow KM, Suardi LZ, Nardin P, Selistre NG, Vázquez D, Gonçalves C-A (2017) Hippocampal changes in STZ-model of Alzheimer’s disease are dependent on sex. Behav Brain Res 316:205–214
Kander MC, Cui Y, Liu Z (2017) Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 21(5):1024–1032
Brunelli E, Domanico F, La Russa D, Pellegrino D (2014) Sex differences in oxidative stress biomarkers. Curr Drug Targets 15(8):811–815
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71(2):621S-629S
Ansari MA, Scheff SW (2010) Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69(2):155–167
Mecocci P, Boccardi V, Cecchetti R, Bastiani P, Scamosci M, Ruggiero C, Baroni M (2018) A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J Alzheimers Dis 62(3):1319–1335
San Tang K (2020) The potential role of nanoyttria in alleviating oxidative stress biomarkers: implications for Alzheimer’s disease therapy. Life Sci. https://doi.org/10.1016/j.lfs.2020.118287
Wojtunik-Kulesza KA, Oniszczuk A, Oniszczuk T, Waksmundzka-Hajnos M (2016) The influence of common free radicals and antioxidants on development of Alzheimer’s Disease. Biomed Pharmacother 78:39–49
Vinitha E, Singh HJ, Kakalij RM, Kshirsagar RP, Kumar BH, Diwan PV (2014) Neuroprotective effect of Prunus avium on streptozotocin induced neurotoxicity in mice. Biomed Prev Nutr 4(4):519–525
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. https://doi.org/10.1155/2013/942916
de Andrade Teles RB, Diniz TC, Costa Pinto TC, de Oliveira Júnior RG, Gama e Silva M, de Lavor ÉM, Fernandes AWC, de Oliveira AP, de Almeida Ribeiro FPR, da Silva AAM (2018) Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev. https://doi.org/10.1155/2018/7043213
Uttara B, Singh AV, Zamboni P, Mahajan R (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74
Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G (2020) Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. https://doi.org/10.1007/s12325-019-01148-5
Bakhtiari M, Panahi Y, Ameli J, Darvishi B (2017) Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed Pharmacother 93:218–229
Kumar S, Mishra A, Pandey AK (2013) Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med 13(1):120
Rop O, Sochor J, Jurikova T, Zitka O, Skutkova H, Mlcek J, Salas P, Krska B, Babula P, Adam V (2011) Effect of five different stages of ripening on chemical compounds in medlar (Mespilus germanica L.). Molecules 16(1):74–91
Kouhestani S, Jafari A, Babaei P (2018) Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen Res 13(10):1827
Pan X, Liu X, Zhao H, Wu B, Liu G (2020) Antioxidant, anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. J Funct Foods 74:104140
Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M (2011) A review on the dietary flavonoid kaempferol. Mini Rev Med Chem 11(4):298–344
Yang E-J, Kim G-S, Jun M, Song K-S (2014) Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct 5(7):1395–1402
Zarei M, Mohammadi S, Jabbari S, Shahidi S (2019) Intracerebroventricular microinjection of kaempferol on memory retention of passive avoidance learning in rats: involvement of cholinergic mechanism (s). Int J Neurosci 129(12):1203–1212
El Sayed NS, Ghoneum MH (2020) Antia, a natural antioxidant product, attenuates cognitive dysfunction in streptozotocin-induced mouse model of sporadic Alzheimer’s disease by targeting the amyloidogenic, inflammatory, autophagy, and oxidative stress pathways. Oxid Med Cell Longev. https://doi.org/10.1155/2020/4386562
Fayaz E, Damirchi A, Zebardast N, Babaei P (2019) Cinnamon extract combined with high-intensity endurance training alleviates metabolic syndrome via non-canonical WNT signaling. Nutrition 65:173–178
Agrawal R, Tyagi E, Shukla R, Nath C (2011) Insulin receptor signaling in rat hippocampus: a study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol 21(3):261–273
Chen J, Gao L, Zhang Y, Su Y, Kong Z, Wang D, Yan M (2020) Acteoside-improved streptozotocin-induced learning and memory impairment by upregulating hippocampal insulin, glucose transport, and energy metabolism. Phytother Res. https://doi.org/10.1002/ptr.6811
Zhou M, Chen S, Peng P, Gu Z, Yu J, Zhao G, Deng Y (2019) Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β. Biochem Biophys Res Commun 511(1):154–160
Dalli T, Beker M, Terzioglu-Usak S, Akbas F, Elibol B (2018) Thymoquinone activates MAPK pathway in hippocampus of streptozotocin-treated rat model. Biomed Pharmacother 99:391–401
Bassani TB, Bonato JM, Machado MM, Cóppola-Segovia V, Moura EL, Zanata SM, Oliveira RM, Vital MA (2018) Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer’s disease in rats. Mol Neurobiol 55(5):4280–4296
Luo H, Xiang Y, Qu X, Liu H, Liu C, Li G, Han L, Qin X (2019) Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat model of Alzheimer’s disease through activation of BDNF-TrkB signaling pathway. Front Pharmacol 10:395
Agrawal M, Perumal Y, Bansal S, Arora S, Chopra K (2020) Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem Toxicol 145:111684
Bao J, Mahaman YA, Liu R, Wang J-Z, Zhang Z, Zhang B, Wang X (2017) Sex differences in the cognitive and hippocampal effects of streptozotocin in an animal model of sporadic AD. Front Aging Neurosci 9:347
Babaei P, Shirkouhi SG, Hosseini R, Tehrani BS (2017) Vitamin D is associated with metabotropic but not neurotrophic effects of exercise in ovariectomized rats. Diabetol Metab Syndr 9(1):1–9
López-Sánchez C, Martín-Romero FJ, Sun F, Luis L, Samhan-Arias AK, García-Martínez V, Gutiérrez-Merino C (2007) Blood micromolar concentrations of kaempferol afford protection against ischemia/reperfusion-induced damage in rat brain. Brain Res 1182:123–137
Li S, Pu X-P (2011) Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol Pharm Bull 34(8):1291–1296
Al-Numair KS, Chandramohan G, Veeramani C, Alsaif MA (2015) Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep 20(5):198–209
El-kott AF, Abd-El-Karim M, Khalifa HS, Morsy K, Ibrahim EH, Bin-Jumah M, Abdel-Daim MM, Aleya L (2020) Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138832
Zhang R, Ai X, Duan Y, Xue M, He W, Wang C, Xu T, Xu M, Liu B, Li C (2017) Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed Pharmacother 89:660–672
Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP (2012) The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav 105(4):1014–1020
Grissom EM, Hawley WR, Hodges KS, Fawcett-Patel JM, Dohanich GP (2013) Biological sex influences learning strategy preference and muscarinic receptor binding in specific brain regions of prepubertal rats. Hippocampus 23(4):313–322
Jones CM, Healy SD (2006) Differences in cue use and spatial memory in men and women. Proc R Soc B 273(1598):2241–2247
Yagi S, Drewczynski D, Wainwright SR, Barha CK, Hershorn O, Galea LA (2017) Sex and estrous cycle differences in immediate early gene activation in the hippocampus and the dorsal striatum after the cue competition task. Horm Behav 1(87):69–79
Funding
This study was supported by a grant from Research and Technology Chancellor of Guilan University of Medical Sciences, Iran (No. IR. GUMS. REC. 1936.51). The funder did not participate in data collection and analysis, paper writing, or submission.
Author information
Authors and Affiliations
Contributions
PB designed this study, performed experiments, wrote and revised the paper for important intellectual content. KE performed experiments and read the manuscript. SK performed experiments, statistical analysis, and participated in paper writing. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
Experiments were conducted according to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and was approved by the ethics committee of Guilan University of Medical Sciences, Rasht, Iran (IR.GUMS.REC.1396.51).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Babaei, P., Eyvani, K. & Kouhestani, S. Sex-Independent Cognition Improvement in Response to Kaempferol in the Model of Sporadic Alzheimer’s Disease. Neurochem Res 46, 1480–1486 (2021). https://doi.org/10.1007/s11064-021-03289-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03289-y