Abstract
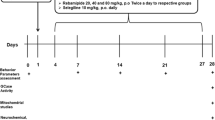
In this study, we were aimed to investigate the neuroprotective effects of bexarotene and nicotinamide in synaptosomes incubated with amyloid-beta (Aβ). Our study consists of 2 parts, in vivo and in vitro. In the in vivo section, twenty-four Wistar albino male rats were divided into 4 groups (control, dimethyl sulfoxide (DMSO), nicotinamide and bexarotene) with six animals in each group. DMSO(1%), nicotinamide(100 mg/kg) and bexarotene(0.1 mg/kg) were administered intraperitoneally to animals in the experimental groups for seven days. In the in vitro part of our study, three different isolation methods were used to obtain the synaptosomes from the brain tissue. Total antioxidant capacity(TAS), total oxidant capacity(TOS), cleaved caspase 3(CASP3), cytochrome c(Cyt c), sirtuin 1(SIRT1), peroxisome proliferator-activated receptor gamma(PPARγ) and poly(ADP-ribose) polymerase-1(PARP-1) levels in the synaptosomes incubated with a concentration of 10 µM Aβ(1-42) were measured by enzyme-linked immunosorbent assay method. Biochemical analysis and histopathological examinations in serum and brain samples showed that DMSO, nicotinamide and bexarotene treatments did not cause any damage to the rat brain tissue. We found that in vitro Aβ(1-42) administration decreased TAS, SIRT1 and PPARγ levels in synaptosomes while increasing TOS, CASP3, Cyt c, and PARP1 levels. Nicotinamide treatment suppressed oxidative stress and apoptosis by supporting antioxidant capacity and increased PPARγ through SIRT1 activation, causing PARP1 to decrease. On the other hand, bexarotene caused a moderate increase in SIRT1 levels with PPARγ activation. Consequently, we found that nicotinamide can be more effective than bexarotene in AD pathogenesis by regulating mitochondrial functions in synaptosomes.







Similar content being viewed by others
References
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis 1:15056. https://doi.org/10.1038/nrdp.2015.56
Tai HC, Wang BY, Serrano-Pozo A, Frosch MP, Spires-Jones TL, Hyman BT (2014) Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol Commun 2(1):146. https://doi.org/10.1186/s40478-014-0146-2
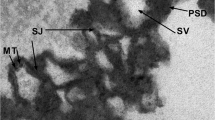
Whittaker VP (1993) Thirty years of synaptosome research. J Neurocytol 22(9):735–742. https://doi.org/10.1007/BF01181319
Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X (2008) Oxidative stress signaling in Alzheimer’s disease. Curr Alzheimer Res 5(6):525–532. https://doi.org/10.2174/156720508786898451
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19(8):823–835. https://doi.org/10.1089/ars.2012.5027
Li Z, Sheng M (2012) Caspases in synaptic plasticity. Mol Brain 5(1):15. https://doi.org/10.1186/1756-6606-5-15
Albani D, Polito L, Forloni G (2010) Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: experimental and genetic evidence. J Alzheimers Dis 19(1):11–26. https://doi.org/10.3233/JAD-2010-1215
Krishnakumar R, Kraus WL (2010) The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 39(1):8–24. https://doi.org/10.1016/j.molcel.2010.06.017
Aredia F, Scovassi AI (2014) Poly (ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol 92(1):157–163. https://doi.org/10.1016/j.bcp.2014.06.021
Yonutas HM, Sullivan PG (2013) Targeting PPAR isoforms following CNS injury. Curr Drug Targets 14(7):733–742. https://doi.org/10.2174/1389450111314070003
Masciopinto F, Di Pietro N, Corona C, Bomba M, Pipino C, Curcio M, Di Castelnuovo A, Ciavardelli D, Silvestri E, Canzoniero LM, Sekler I, Pandolfi A, Sensi SL (2012) Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death Dis 3(12):e448. https://doi.org/10.1038/cddis.2012.189
Gniadecki R, Assaf C, Bagot M, Dummer R, Duvic M, Knobler R, Ranki A, Schwandt P, Whittaker S (2007) The optimal use of bexarotene in cutaneous T-cell lymphoma. Br J Dermatol 157(3):433–440. https://doi.org/10.1111/j.1365-2133.2007.07975.x
Cramer PE, Cirrito JR, Wesson DW, Lee CD, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE (2012) ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Sci 335(6075):1503–1506. https://doi.org/10.1126/science
Tai LM, Koster KP, Luo J, Lee SH, Wang YT, Collins NC, Ben Aissa M, Thatcher GR, LaDu MJ (2014) Amyloid-β pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. Journal of Biological Chem 289(44):30538–30555. https://doi.org/10.1074/jbc.M114.600833
Huy PDQ, Thai NQ, Bednarikova Z, Phuc LH, Linh HQ, Gazova Z, Li MS (2017) Bexarotene does not clear amyloid beta plaques but delays fibril growth: Molecular mechanisms. ACS chemical neuroscience 8(9):1960–1969. https://doi.org/10.1021/acschemneuro.7b00107
Belenky P, Bogan KL, Brenner C (2007) NAD+ metabolism in health and disease. Trends Biochem Sci 32(1):12–19. https://doi.org/10.1016/j.tibs.2006.11.006
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM (2008) Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci 28(45):11500–11510. https://doi.org/10.1523/JNEUROSCI.3203-08.2008
Bayrakdar ET, Armagan G, Uyanikgil Y, Kanit L, Koylu E, Yalcin A (2014) Ex vivo protective effects of nicotinamide and 3-aminobenzamide on rat synaptosomes treated with Aβ (1–42). Cell Biochem Funct 32(7):557–564. https://doi.org/10.1002/cbf.3049
Tunctan B, Kucukkavruk SP, Temiz-Resitoglu M, Guden DS, Sari AN, Sahan-Firat S (2018) Bexarotene, a selective RXRα agonist, reverses hypotension associated with inflammation and tissue injury in a rat model of septic shock. Inflammation 41(1):337–355. https://doi.org/10.1007/s10753-017-0691-5
Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D (2006) APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38(1):24–26. https://doi.org/10.1038/ng1718
Whittaker VP, Michaelson I, Kirkland RJA (1964) The separation of synaptic vesicles from nerve-ending particles (synaptosomes’). Biochem J 90(2):293. https://doi.org/10.1042/bj0900293
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein Measurement with the Folin-Phenol Reagent. J Biol Chem 193(1):265–375
Tenreiro P, Rebelo S, Martins F, Santos M, Coelho ED, Almeida M, Matos AAD, e Silva ODC (2017) Comparison of simple sucrose and percoll based methodologies for synaptosome enrichment. Anal Biochem 517:1–8. https://doi.org/10.1016/j.ab.2016.10.015
Sokolow S, Henkins KM, Williams IA, Vinters HV, Schmid I, Cole GM, Gylys KH (2012) Isolation of synaptic terminals from Alzheimer’s disease cortex. Cytometry Part A 81(3):248–254. https://doi.org/10.1002/cyto.a.22009
Dunkley PR, Jarvie PE, Robinson PJ (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3(11):1718. https://doi.org/10.1038/nprot.2008.171
Dodd PR, Hardy JA, Oakley AE, Edwardson JA, Perry EK, Delaunoy JP (1981) A rapid method for preparing synaptosomes: comparison, with alternative procedures. Brain Res 226(1–2):107–118. https://doi.org/10.1016/0006-8993(81)91086-6
Sherman AD (1989) Isolation of metabolically distinct synaptosomes on Percoll gradients. Neurochem Res 14(1):97–101. https://doi.org/10.1007/bf00969765
Bai F, Witzmann FA (2007) Synaptosome proteomics. Subcell Biochem 3:77–98. https://doi.org/10.1007/978-1-4020-5943-8_6
Mattson MP, Liu D (2002) Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med 2(2):215–231. https://doi.org/10.1385/NMM:2:2:215
Pocernich CB (1822) Butterfield DA (2012) Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 5:625–630. https://doi.org/10.1016/j.bbadis.2011.10.003
Pratico D (2008) Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Neurosci 29(12):609–615. https://doi.org/10.1016/j.tips.2008.09.001
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH (2010) Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20(2):609–631. https://doi.org/10.3233/JAD-2010-100564
Hroudova J, Singh N, Fisar Z (2014) Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. Biomed Res Int 2014:175062. https://doi.org/10.1155/2014/175062
Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE (2008) Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathol 173(5):1488–1495. https://doi.org/10.2353/ajpath.2008.080434
Ng F, Wijaya L, Tang BL (2015) SIRT1 in the brain—connections with aging-associated disorders and lifespan. Front Cell Neurosci 9:64. https://doi.org/10.3389/fncel.2015.00064
Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y (2007) Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282(9):6823–6832. https://doi.org/10.1074/jbc.M609554200
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281(31):21745–21754. https://doi.org/10.1074/jbc.M602909200
Julien C, Tremblay C, Emond V, Lebbadi M, JrN S, Bennett DA, Calon F (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp 68(1):48–58. https://doi.org/10.1097/NEN.0b013e3181922348
Schmitt K, Grimm A, Kazmierczak A, Strosznajder JB, Götz J, Eckert A (2012) Insights into mitochondrial dysfunction: aging, amyloid-β, and tau–a deleterious trio. Antioxid Redox Signal 16(12):1456–1466. https://doi.org/10.1089/ars.2011.4400
Strosznajder JB, Czapski GA, Adamczyk A, Strosznajder RP (2012) Poly (ADP-ribose) polymerase-1 in amyloid beta toxicity and Alzheimer’s disease. Mol Neurobiol 46(1):78–84. https://doi.org/10.1007/s12035-012-8258-9
de la Monte SM, Wands JR (2006) Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimer’s Dis 9(2):167–181. https://doi.org/10.3233/jad-2006-9209
Moosecker S, Gomes PA, Dioli C, Yu S, Sotiropoulos I, Almeida OF (2019) Activated PPARγ abrogates misprocessing of amyloid precursor protein, Tau missorting and synaptotoxicity. Front Cell Neurosci 13:239. https://doi.org/10.3389/fncel.2019.00239
Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M (2005) Peroxisome proliferator-activated receptor γ is expressed in hippocampal neurons and its activation prevents β-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res 304(1):91–104. https://doi.org/10.1016/j.yexcr.2004.09.032
Bieganowski P, Brenner C (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117(4):495–502. https://doi.org/10.1016/s0092-8674(04)00416-7
Demarin V, Podobnik SS, Storga-Tomic D, Kay G (2004) Treatment of Alzheimer’s disease with stabilized oral nicotinamide adenine dinucleotide: a randomized, double-blind study. Drugs Exp Clin Res 30(1):27–33
Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP (2009) Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromol Med 11(1):28–42. https://doi.org/10.1007/s12017-009-8058-1
Li X, Zhang S, Blander G, Jeanette GT, Krieger M, Guarente L (2007) SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 28(1):91–106. https://doi.org/10.1016/j.molcel.2007.07.032
Martire S, Fuso A, Rotili D, Tempera I, Giordano C, De Zottis I, Muzi A, Vernole P, Graziani G, Lococo E, Faraldi M, Maras B, Scarpa S, Mosca L, d’Erme M (2013) PARP-1 modulates amyloid beta peptide-induced neuronal damage. PLoS ONE. https://doi.org/10.1371/journal.pone.0072169
Smith BC, Hallows WC, Denu JM (2009) A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal Biochem 394(1):101–109. https://doi.org/10.1016/j.ab.2009.07.019
Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13(4):461–468. https://doi.org/10.1016/j.cmet.2011.03.004
Kuntz M, Candela P, Saint-Pol J, Lamartiniere Y, Boucau MC, Sevin E, Fenart L, Gosselet F (2015) Bexarotene promotes cholesterol efflux and restricts apical-to-basolateral transport of amyloid-β peptides in an in vitro model of the human blood-brain barrier. J Alzheimer’s Dis 48(3):849–862. https://doi.org/10.3233/JAD-150469
Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT (2006) Nonsteroidal anti-inflammatory drugs repress β-secretase gene promoter activity by the activation of PPARγ. Proc Natl Acad Sci USA 103(2):443–448. https://doi.org/10.1073/pnas.0503839103
Ghosal K, Haag M, Verghese PB, West T, Veenstra T, Braunstein JB, Bateman RJ, Holtzman DM, Landreth GE (2016) A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement (NY) 2(2):110–120. https://doi.org/10.1016/j.trci.2016.06.001
Hwang ES, Song SB (2020) Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules 10(5):687. https://doi.org/10.3390/biom10050687
Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC, Bexarotene Worldwide Study Group (2001) Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 19(9):2456–71. https://doi.org/10.1200/JCO.2001.19.9.2456
Cramer PE, Cirrito JR, Wesson DW, Lee CD, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE (2012) ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335(6075):1503–1506. https://doi.org/10.1126/science
Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T (2010) SIRT1 is regulated by a PPARγ–SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res 38(21):7458–7471. https://doi.org/10.1093/nar/gkq609
Funding
This study was supported by Eskişehir Osmangazi University Scientific Research Commission with grant number 201811D21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kar, F. & Kanbak, G. Ex Vivo Investigation of Bexarotene and Nicotinamide Function as a Protectıve Agent on Rat Synaptosomes Treated with Aβ(1-42). Neurochem Res 46, 804–818 (2021). https://doi.org/10.1007/s11064-020-03216-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03216-7