Abstract
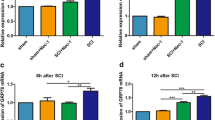
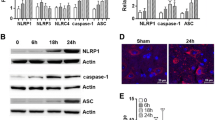
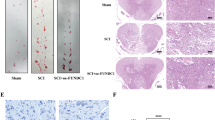
Spinal cord injury (SCI) is one of the diseases with high probability of causing disability in human beings, and there is no reliable treatment at present. Neuronal apoptosis is a vital component of secondary injury and plays a critical role in the development of neurological dysfunction after spinal cord injury. In this study, we found that the expression and distribution of HAX-1 in neurons increased 1 day after SCI. PC12 cells overexpressing HAX-1 showed decreased apoptosis and PC12 cells are more likely to undergo apoptosis after down-regulating HAX-1, which was confirmed via TUNEL experiments. We found GRP94 showed the same trend as HAX-1 in expression and interacted with HAX-1 and IRE-1 in both spinal cord tissue and PC12 cells, and this interaction seems to be enhanced after SCI. When the expression of HAX-1 was up-regulated, GRP94 also increased, but IRE-1 did not change at all. Further studies showed that overexpression of HAX-1 decreased the expression of pIRE-1, rather than IRE-1, and downstream proteins of the IRE signaling pathway (Caspase12, pJNK and CHOP) were significantly reduced, and vice versa. In animals treated with HAX-1 expressing adenovirus there are more neuronal cells remaining in the damaged spinal cord tissue, and hindlimb motor function of rats was significantly improved. So, we speculate that HAX-1 might play a role in protecting neurons from apoptosis after SCI by regulating the IRE-1 signaling pathway via promoting the dissociation of GRP94 from IRE-1. This may provide a theoretical basis and a potential therapeutic target for clinical improvement of neural function recovery after SCI.




Similar content being viewed by others
References
Casper DS, Zmistowski B, Schroeder GD, McKenzie JC, Mangan J, Vatson J, Hilibrand AS, Vaccaro AR, Kepler CK (2018) Preinjury patient characteristics and postinjury neurological status are associated with mortality following spinal cord injury. Spine (Phila Pa 1976) 43:895–899
Byra S (2016) Posttraumatic growth in people with traumatic long-term spinal cord injury: predictive role of basic hope and coping. Spinal Cord 54:478–482
Nagoshi N, Kaneko S, Fujiyoshi K, Takemitsu M, Yagi M, Iizuka S, Miyake A, Hasegawa A, Machida M, Konomi T, Machida M, Asazuma T, Nakamura M (2016) Characteristics of neuropathic pain and its relationship with quality of life in 72 patients with spinal cord injury. Spinal Cord 54:656–661
Lee DY, Park YJ, Song SY, Hwang SC, Kim KT, Kim DH (2018) The importance of early surgical decompression for acute traumatic spinal cord injury. Clin Orthop Surg 10:448–454
Ahuja CS, Martin AR, Fehlings M (2016) Recent advances in managing a spinal cord injury secondary to trauma. F1000Res 5:F1000
Lu X, Xue P, Fu L, Zhang J, Jiang J, Guo X, Bao G, Xu G, Sun Y, Chen J, Cui Z (2018) HAX1 is associated with neuronal apoptosis and astrocyte proliferation after spinal cord injury. Tissue Cell 54:1–9
Deng X, Song L, Zhao W, Wei Y, Guo XB (2017) HAX-1 protects glioblastoma cells from apoptosis through the Akt1 pathway. Front Cell Neurosci 11:420
Yan J, Ma C, Cheng J, Li Z, Liu C (2015) HAX-1 inhibits apoptosis in prostate cancer through the suppression of caspase-9 activation. Oncol Rep 34:2776–2781
Guo XB, Deng X, Wei Y (2018) Hematopoietic substrate-1-associated protein X-1 regulates the proliferation and apoptosis of endothelial progenitor cells through akt pathway modulation. Stem Cells 36:406–419
Lam CK, Zhao W, Cai W, Vafiadaki E, Florea SM, Ren X, Liu Y, Robbins N, Zhang Z, Zhou X, Jiang M, Rubinstein J, Jones WK, Kranias EG (2013) Novel role of HAX-1 in ischemic injury protection involvement of heat shock protein 90. Circ Res 112:79–89
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940
Matsuyama D, Watanabe M, Suyama K, Kuroiwa M, Mochida J (2014) Endoplasmic reticulum stress response in the rat contusive spinal cord injury model-susceptibility in specific cell types. Spinal Cord 52:9–16
Sakurai M, Takahashi G, Abe K, Horinouchi T, Itoyama Y, Tabayashi K (2005) Endoplasmic reticulum stress induced in motor neurons by transient spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg 130:640–645
Ohri SS, Maddie MA, Zhang Y, Shields CB, Hetman M, Whittemore SR (2012) Deletion of the pro-apoptotic endoplasmic reticulum stress response effector CHOP does not result in improved locomotor function after severe contusive spinal cord injury. J Neurotrauma 29:579–588
Wu CT, Weng TI, Chen LP, Chiang CK, Liu SH (2013) Involvement of caspase-12-dependent apoptotic pathway in ionic radiocontrast urografin-induced renal tubular cell injury. Toxicol Appl Pharmacol 266:167–175
Rathore AP, Ng ML, Vasudevan SG (2013) Differential unfolded protein response during Chikungunya and Sindbis virus infection: CHIKV nsP4 suppresses eIF2alpha phosphorylation. Virol J 10:36
Badiola N, Penas C, Minano-Molina A, Barneda-Zahonero B, Fado R, Sanchez-Opazo G, Comella JX, Sabria J, Zhu C, Blomgren K, Casas C, Rodriguez-Alvarez J (2011) Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis 2:e149
Guo G, Meng Y, Tan W, Xia Y, Cheng C, Chen X, Gu Z (2015) Induction of apoptosis coupled to endoplasmic reticulum stress through regulation of CHOP and JNK in bone marrow mesenchymal stem cells from patients with systemic lupus erythematosus. J Immunol Res 2015:183738
Wu C, Cui Z, Liu Y, Zhang J, Ding W, Wang S, Bao G, Xu G, Sun Y, Chen J (2016) The importance of EHD1 in neurite outgrowth contributing to the functional recovery after spinal cord injury. Int J Dev Neurosci 52:24–32
Short B (2015) TUNEL vision spots apoptotic cells. J Cell Biol 208:7–7
Montenegro-Miranda PS, Pichard V, Aubert D, ten Bloemendaal L, Duijst S, de Waart DR, Ferry N, Bosma PJ (2014) In the rat liver, Adenoviral gene transfer efficiency is comparable to AAV. Gene Ther 21:168–174
Song YH, Agrawal NK, Griffin JM, Schmidt CE (2019) Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev 148:38–59
James ND, McMahon SB, Field-Fote EC, Bradbury EJ (2018) Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol 17:905–917
Tran AP, Warren PM, Silver J (2018) The biology of regeneration failure and success after spinal cord injury. Physiol Rev 98:881–917
Funding
This study was supported by National Natural Science Foundation of China (Grant Numbers: 81771319, 81501076, 81702216), Nantong Health and Family Planning Commission Research Project (Grant Numbers: WKZL2018001; MSZ18209), Nantong Science and Technology Plan Project (Grant Numbers: JCZ18137), the ‘Top Six Types of Talents’ Financial Assistance of Jiangsu Province Grant (WSW-156) and Construction of Trauma Emergency Medical Research Center (Grant Numbers: HS2018002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Yang, S., Wu, C. et al. Novel Role of HAX-1 in Neurons Protection After Spinal Cord Injury Involvement of IRE-1. Neurochem Res 45, 2302–2311 (2020). https://doi.org/10.1007/s11064-020-03088-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03088-x