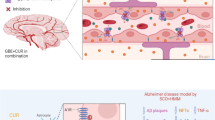
Abstract
Safflower yellow (SY) is the main effective component of Carthamus tinctorius L., and Hydroxysafflor yellow A (HSYA) is the single active component with the highest content in SY. SY and HSYA have been shown to have neuroprotective effects in several AD models. In this study, we aimed to clarify whether the effects of SY and HSYA on the learning and memory abilities of Aβ1-42-induced AD model rats are related to the enhancement of synaptic structural plasticity in brain tissues and the amelioration of disorder of glutamate circulation. We used rats injected with Aβ1-42 into the bilateral hippocampus as a model of AD. After treatment with SY and HSYA, the learning and memory abilities of the Aβ1-42-induced AD model rats were enhanced, Aβ deposition in the AD model rats was decreased, structural damage to dendritic spines and the loss of synaptic-associated proteins were alleviated, and the disorder of glutamate circulation was ameliorated. The results indicated that SY and HSYA improve synaptic structural plasticity by ameliorating the disorder of glutamate circulation in Aβ1-42-induced AD model rats.







Similar content being viewed by others
References
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Godyń J, Jończyk J, Panek D, Malawska B (2016) Therapeutic strategies for Alzheimer's disease in clinical trials. Pharmacol Rep 68:127–138. https://doi.org/10.1016/j.pharep.2015.07.006
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D (2009) Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62:788–801. https://doi.org/10.1016/j.neuron.2009.05.012
O'Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 34:185–204. https://doi.org/10.1146/annurev-neuro-061010-113613
Irie Y, Keung WM (2003) Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-β peptide. Brain Res 963:282–289. https://doi.org/10.1016/s0006-8993(02)04050-7
Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658
Varga E, Juhász G, Bozsó Z, Penke B, Fülöp L, Szegedi V (2015) Amyloid-β1-42 disrupts synaptic plasticity by altering glutamate recycling at the synpse. J Alzheimer's dis 45(449):456. https://doi.org/10.3233/JAD-142367
Seo J, Giusti-Rodríguez P, Zhou Y, Rudenko A, Cho S, Ota KT, Park C, Patzke H, Madabhushi R, Pan L, Mungenast AE, Guan J-S, Delalle I, Tsai L-H (2014) Activity-dependent p25 generation regulates synaptic plasticity and Aβ-induced cognitive impairment. Cell 157:486–498. https://doi.org/10.1016/j.cell.2014.01.065
Arias C, Arrieta I, Tapia R (1995) β-Amyloid peptide fragment 25–35 potentiates the calcium-dependent release of excitatory amino acids from depolarized hippocampal slices. J Neurosci Res 41:561–566. https://doi.org/10.1002/jnr.490410416
Chen L, Xiang Y, Kong L, Zhang X, Sun B, Wei X, Liu H (2013) Hydroxysafflor yellow a protects against cerebral ischemia–reperfusion injury by anti-apoptotic effect through PI3K/Akt/GSK3β pathway in rat. Neurochem Res 38:2268–2275. https://doi.org/10.1007/s11064-013-1135-8
Pan Y, Zheng DY, Liu SM, Meng Y, Xu HY, Zhang Q, Gong J, Xia ZL, Chen LB, Li HY (2012) Hydroxysafflor yellow A attenuates lymphostatic encephalopathy-induced brain injury in rats. Phytother Res 26:1500–1506
Zhang L, Zhou Z, Zhai W, Pang J, Mo Y, Yang G, Qu Z, Hu Y (2019) Safflower yellow attenuates learning and memory deficits in amyloid β-induced Alzheimer’s disease rats by inhibiting neuroglia cell activation and inflammatory signaling pathways. Metab Brain Dis 34:927–939. https://doi.org/10.1007/s11011-019-00398-0
Shi X-m, Zhang H, Zhou Z-j, Ruan Y-y, Pang J, Zhang L, Zhai W, Hu Y-l (2018) Effects of safflower yellow on beta-amyloid deposition and activation of astrocytes in the brain of APP/PS1 transgenic mice. Biomed Pharmacother 98:553–565. https://doi.org/10.1016/j.biopha.2017.12.099
Ruan Y-y, Zhai W, Shi X-m, Zhang L, Hu Y-l (2016) Safflower yellow ameliorates cognition deficits and reduces tau phosphorylation in APP/PS1 transgenic mice. Metab Brain Dis 31(5):1133–1142. https://doi.org/10.1007/s11011-016-9857-3
Ma Q, Ruan Y-y, Xu H, Shi X-m, Wang Z-x, Hu Y-l (2015) Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid β-induced impairment of learning and memory in rats. Biomed Pharmacother 76:153–164. https://doi.org/10.1016/j.biopha.2015.10.004
Sun Y, Xu D-P, Qin Z, Wang P-Y, Hu B-H, Yu J-G, Zhao Y, Cai B, Chen Y-L, Lu M (2018) Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur J Pharmacol 818:604–609. https://doi.org/10.1016/j.ejphar.2017.11.033
Chen S, Sun M, Zhao X, Yang Z, Liu W, Cao J, Qiao Y, Luo X, Wen A (2019) Neuroprotection of hydroxysafflor yellow A in experimental cerebral ischemia/reperfusion injury via metabolic inhibition of phenylalanine and mitochondrial biogenesis. Mol Med Rep 19:3009–3020. https://doi.org/10.3892/mmr.2019.9959
Zhang Z-h, Yu L-j, Hui X-c, Wu Z-z, Yin K-l, Yang H, Xu Y (2014) Hydroxy-safflor yellow A attenuates Aβ1-42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res 1563:72–80. https://doi.org/10.1016/j.brainres.2014.03.036
Yang Q, Yang Z-F, Liu S-B, Zhang X-N, Hou Y, Li X-Q, Wu Y-M, Wen A-D, Zhao M-G (2010) Neuroprotective effects of hydroxysafflor yellow A against excitotoxic neuronal death partially through down-regulation of NR2B-containing NMDA receptors. Neurochem Res 35:1353–1360. https://doi.org/10.1007/s11064-010-0191-6
Ghumatkar P, Peshattiwar V, Patil S, Muke S, Whitfield D, Howlett D, Francis P, Sathaye S (2018) The effect of phloretin on synaptic proteins and adult hippocampal neurogenesis in Aβ (1–42)-injected male Wistar rats. J Pharm Pharmacol 70:1022–1030. https://doi.org/10.1111/jphp.12925
Chen J-H, Ke K-F, Lu J-H, Qiu Y-H, Peng Y-P (2015) Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1–42-induced Alzheimer’s disease model rats. PLoS ONE 10:e0116549. https://doi.org/10.1371/journal.pone.0116549
Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13:1676–1687. https://doi.org/10.1523/JNEUROSCI.13-04-01676.1993
Shen W-X, Chen J-H, Lu J-H, Peng Y-P, Qiu Y-H (2014) TGF-β1 protection against Aβ1-42-induced neuroinflammation and neurodegeneration in rats. Int J Mol Sci 15:22092–22108. https://doi.org/10.3390/ijms151222092
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Di Marzo V, Drago F (2010) Enhanced cognitive performance of dopamine D3 receptor “knock-out” mice in the step-through passive-avoidance test: assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol Res 61:531–536. https://doi.org/10.1016/j.phrs.2010.02.003
Kameyama T, Nabeshima T, Kozawa T (1986) Step-down-type passive avoidance-and escape-learning method: suitability for experimental amnesia models. J Pharmacol methods 16:39–52. https://doi.org/10.1016/0160-5402(86)90027-6
Zhang H, Lai Q, Li Y, Liu Y, Yang M (2017) Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol 196:225–235. https://doi.org/10.1016/j.jep.2016.11.042
Khurana R, Coleman C, Ionescu-Zanetti C, Carter SA, Krishna V, Grover RK, Roy R, Singh S (2005) Mechanism of thioflavin T binding to amyloid fibrils. J Struct Biol 151:229–238. https://doi.org/10.1016/j.jsb.2005.06.006
Waldvogel HJ, Curtis MA, Baer K, Rees MI, Faull RL (2006) Immunohistochemical staining of post-mortem adult human brain sections. Nat Protoc 1:2719–2732. https://doi.org/10.1038/nprot.2006.354
Du F (2019) Golgi-Cox staining of neuronal dendrites and dendritic spines with FD rapid GolgiStain™ kit. Curr Protoc Neurosci 88:e69. https://doi.org/10.1002/cpns.69
Avila JA, Alliger AA, Carvajal B, Zanca RM, Serrano PA, Luine VN (2017) Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27:1224–1229. https://doi.org/10.1002/hipo.22768
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8(6):595–608
Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–186. https://doi.org/10.1126/science.1566067
Nimmrich V, Ebert U (2009) Is Alzheimer's disease a result of presynaptic failure?-Synaptic dysfunctions induced by oligomeric β-amyloid. Rev Neurosci 20:1–12. https://doi.org/10.1515/REVNEURO.2009.20.1.1
Yao N, Li J, Liu H, Wan J, Liu W, Zhang M (2017) The structure of the ZMYND8/Drebrin complex suggests a cytoplasmic sequestering mechanism of ZMYND8 by Drebrin. Structure 25:1657–1666. https://doi.org/10.1016/j.str.2017.08.014
Noguchi J, MatsuzakiM E-D, Kasai H (2005) Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46:609–622. https://doi.org/10.1016/j.neuron.2005.03.015
Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y (2004) Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol 468:86–95. https://doi.org/10.1002/cne.10950
Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17:381–386. https://doi.org/10.1016/j.conb.2007.04.009
González-Tapia D, Velázquez-Zamora DA, Olvera-Cortés ME, González-Burgos I (2015) The motor learning induces plastic changes in dendritic spines of Purkinje cells from the neocerebellar cortex of the rat. Restor Neurol Neurosci 33:639–645. https://doi.org/10.3233/RNN-140462
Schaefer N, Rotermund C, Blumrich EM, Lourenco MV, Joshi P, Hegemann RU, Jamwal S, Ali N, Garcia Romero EM, Sharma S (2017) The malleable brain: plasticity of neural circuits and behavior–a review from students to students. J Neurochem 142:790–811. https://doi.org/10.1111/jnc.14107
Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM, Boxer A, Miller BL (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J 30:4141–4148. https://doi.org/10.1096/fj.201600816R
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S-i, Dziewczapolski G, Nakamura T, Cao G, Pratt AE (2013) Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci 110:E2518–E2527. https://doi.org/10.1073/pnas.1306832110
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634. https://doi.org/10.1016/0896-6273(88)90162-6
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105. https://doi.org/10.1016/S0301-0082(00)00067-8
Lu YQ, Luo Y, He ZF, Chen J, Yan BL, Wang Y, Yu Q (2013) Hydroxysafflor yellow A ameliorates homocysteine-induced Alzheimer-like pathologic dysfunction and memory/synaptic disorder. Rejuvenation Res 16:446–452. https://doi.org/10.1089/rej.2013.1451
Vassar PS, Culling C (1959) Fluorescent stains, with special reference to amyloid and connective tissues. Arch Pathol 68:487–498
Acknowledgements
This research was supported by the National Natural Science Foundation of China [No. 81660603 and No. 81960665]. Thanks to American Journal Experts for providing language help in this article (Certificate Verification Key: 3276-319C-8B2C-DF79-341F).
Author information
Authors and Affiliations
Contributions
JH and YH conceived and designed research. CW and JH conducted experiments. MZ, MR and JH analyzed data. JH wrote the manuscript; GY, ZQ and YH revised the manuscript. All authors read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Animals
The animals were treated according to the guidelines of the Animal Management Rules of the Ministry of Health of the People’s Republic of China (documentation Number 55, 2001, Ministry of Health of PR China). The experiments were approved by the Committee of Experimental Animal Administration of Shihezi University.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, J., Wang, C., Zhang, M. et al. Safflower Yellow Improves the Synaptic Structural Plasticity by Ameliorating the Disorder of Glutamate Circulation in Aβ1-42-induced AD Model Rats. Neurochem Res 45, 1870–1887 (2020). https://doi.org/10.1007/s11064-020-03051-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03051-w