Abstract
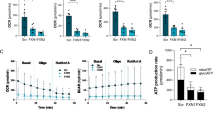
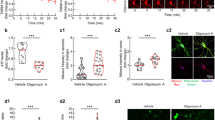
Calpains are calcium-dependent proteases activated in apoptotic cell death and neurodegeneration. Friedreich Ataxia is a neurodegenerative rare disease caused by frataxin deficiency, a mitochondrial protein. Dorsal root ganglion (DRG) sensory neurons are among the cellular types most affected in this disease. We have previously demonstrated that frataxin-deficient DRGs show calpain activation, alteration in calcium levels and decreased content of the Na+/Ca2+ exchanger (NCLX). This transporter is involved in mitochondrial calcium efflux. In this study, we have performed a time-course analysis of several parameters altered in a frataxin-deficient DRGs. These include decline of NCLX levels, calcium accumulation, mitochondrial depolarization, α-fodrin fragmentation and apoptotic cell death. Furthermore, we have analysed the effect of the calpain inhibitors MDL28170 and Calpeptin on these parameters. We have observed that these inhibitors increase NCLX levels, protect sensory neurons from neurite degeneration and calcium accumulation, and restore mitochondrial membrane potential. In addition, calpain 1 reduction alleviated neurodegeneration in frataxin-deficient DRG neurons. These results strengthen the hypothesis of a central role for calcium homeostasis and calpains in frataxin-deficient dorsal root ganglia neurons.








Similar content being viewed by others
References
Campuzano V, Montermini L, Molto MD et al (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427
González-Cabo P, Palau F (2013) Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem 126:53–64. https://doi.org/10.1111/jnc.12303
Stemmler TL, Lesuisse E, Pain D, Dancis A (2010) Frataxin and Mitochondrial FeS Cluster Biogenesis. J Biol Chem 285:26737–26743. https://doi.org/10.1074/jbc.R110.118679
Mincheva-Tasheva S, Obis E, Tamarit J, Ros J (2014) Apoptotic cell death and altered calcium homeostasis caused by frataxin depletion in dorsal root ganglia neurons can be prevented by BH4 domain of Bcl-xL protein. Hum Mol Genet 23:1829–1841. https://doi.org/10.1093/hmg/ddt576
Purroy R, Britti E, Delaspre F et al (2018) Mitochondrial pore opening and loss of Ca2 + exchanger NCLX levels occur after frataxin depletion. Biochim Biophys Acta—Mol Basis Dis 1864:618–631. https://doi.org/10.1016/j.bbadis.2017.12.005
Singh R, Brewer MK, Mashburn CB et al (2014) Calpain 5 is highly expressed in the central nervous system (CNS), carries dual nuclear localization signals, and is associated with nuclear promyelocytic leukemia protein bodies. J Biol Chem 289:19383–19394. https://doi.org/10.1074/jbc.M114.575159
Geddes JW (2013) Mitochondrial Calpains: who, what, where, when and why? Proteases in health and disease. Springer New York, New York, pp 21–32
Samanta K, Kar P, Ghosh B et al (2007) Localization of m-calpain and calpastatin and studies of their association in pulmonary smooth muscle endoplasmic reticulum. Biochim Biophy Acta (BBA)—Gen Subj 1770:1297–1307. https://doi.org/10.1016/j.bbagen.2007.06.010
Shintani-Ishida K, Yoshida K (2015) Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia–reperfusion. Int J Cardiol 197:26–32. https://doi.org/10.1016/J.IJCARD.2015.06.010
Camins A, Verdaguer E, Folch J, Pallàs M (2006) Involvement of Calpain activation in neurodegenerative processes. CNS Drug Rev 12:135–148. https://doi.org/10.1111/j.1527-3458.2006.00135.x
Atherton J, Kurbatskaya K, Bondulich M et al (2014) Calpain cleavage and inactivation of the sodium calcium exchanger-3 occur downstream of Aβ in Alzheimer’s disease. Aging Cell 13:49–59. https://doi.org/10.1111/acel.12148
Bano D, Young KW, Guerin CJ et al (2005) Cleavage of the plasma membrane Na+/Ca2 + exchanger in excitotoxicity. Cell 120:275–285. https://doi.org/10.1016/j.cell.2004.11.049
Villalpando Rodriguez GE, Torriglia A (2013) Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta (BBA)—Mol Cell Res 1833:2244–2253. https://doi.org/10.1016/J.BBAMCR.2013.05.019
Tsujinaka T, Kajiwara Y, Kambayashi J et al (1988) Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem Biophys Res Commun 153:1201–1208. https://doi.org/10.1016/S0006-291X(88)81355-X
Donkor IO (2015) An updated patent review of calpain inhibitors (2012–2014). Expert Opin Ther Pat 25:17–31. https://doi.org/10.1517/13543776.2014.982534
Chard PS, Bleakman D, Savidge JR, Miller RJ (1995) Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience 65:1099–1108. https://doi.org/10.1016/0306-4522(94)00548-j
Kar P, Chakraborti T, Samanta K, Chakraborti S (2009) µ-Calpain mediated cleavage of the Na+/Ca2 + exchanger in isolated mitochondria under A23187 induced Ca2 + stimulation. Arch Biochem Biophys 482:66–76. https://doi.org/10.1016/J.ABB.2008.11.024
Britti E, Delaspre F, Feldman A et al (2018) Frataxin-deficient neurons and mice models of Friedreich ataxia are improved by TAT-MTScs-FXN treatment. J Cell Mol Med 22:834–848. https://doi.org/10.1111/jcmm.13365
de la Fuente S, Sansa A, Periyakaruppiah A et al (2019) Calpain inhibition increases SMN protein in spinal cord motoneurons and ameliorates the spinal muscular atrophy phenotype in mice. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1379-z
Gandhi S, Wood-Kaczmar A, Yao Z et al (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Molecular cell 33:627–638. https://doi.org/10.1016/j.molcel.2009.02.013
Angelova PR, Vinogradova D, Neganova ME et al (2019) Pharmacological sequestration of mitochondrial calcium uptake protects neurons against glutamate excitotoxicity. Mol Neurobiol 56:2244–2255. https://doi.org/10.1007/s12035-018-1204-8
Ludtmann MHR, Kostic M, Horne A et al (2019) LRRK2 deficiency induced mitochondrial Ca 2 + efflux inhibition can be rescued by Na + /Ca 2+ /Li + exchanger upregulation. Cell Death Dis. https://doi.org/10.1038/s41419-019-1469-5
Kostic M, Ludtmann MHR, Bading H et al (2015) PKA Phosphorylation of NCLX reverses mitochondrial calcium overload and depolarization, promoting survival of PINK1-deficient dopaminergic neurons. Cell Rep 13:376–386. https://doi.org/10.1016/j.celrep.2015.08.079
Nath R, Raser KJ, Stafford D et al (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319(Pt 3):683–690. https://doi.org/10.1042/bj3190683
Siman R, Baudry M, Lynch G (1984) Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Nat Acad Sci 81:3572–3576. https://doi.org/10.1073/pnas.81.11.3572
Palty R, Silverman WF, Hershfinkel M et al (2010) NCLX is an essential component of mitochondrial Na+/Ca2 + exchange. Proc Nat Acad Sci 107:436–441. https://doi.org/10.1073/pnas.0908099107
Palomo GM, Cerrato T, Gargini R, Diaz-Nido J (2011) Silencing of frataxin gene expression triggers p53-dependent apoptosis in human neuron-like cells. Hum Mol Genet 20:2807–2822. https://doi.org/10.1093/hmg/ddr187
Acknowledgements
This work has been funded by Project SAF2017-83883-R from MINECO (Spain), the Juan de la Cierva Incorporación postdoctoral fellowship to FD. We thank Arabela Sanz for helping with DRG isolation and processing and Roser Pané for technical assistance. We are indebted to R. Soler lab for kindly supplying lentivirus carrying shCALP1 and EV and for Calpain 1 antibody.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Prof Juan Bolanos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Britti, E., Delaspre, F., Tamarit, J. et al. Calpain-Inhibitors Protect Frataxin-Deficient Dorsal Root Ganglia Neurons from Loss of Mitochondrial Na+/Ca2+ Exchanger, NCLX, and Apoptosis. Neurochem Res 46, 108–119 (2021). https://doi.org/10.1007/s11064-020-03020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03020-3