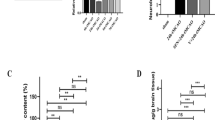
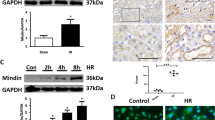
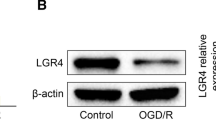
Abstract
Blood brain barrier (BBB) disruption plays an important role in brain injury after acute kidney injury (AKI). However, its underlying mechanisms remain poorly understood. Recent evidence has revealed that proper mitochondrial function is essential for BBB permeability. Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is a key factor in mitochondrial biogenesis and function. This study was designed to investigate the role of PGC-1α in BBB injury after AKI and its related mechanisms. Mice received recombinant adenovirus encoding murine PGC-1α (100 μl, 1.0 × 109PFU/ml) or vehicle 5 days before renal I/R or sham operation. Twenty-four hours after the operation, brain, kidney and serum samples were collected for assessments. We found that mice suffering from renal I/R injury showed decreased PGC-1α levels in both the kidney and BBB. PGC-1α transfection resulted in increased PGC-1α level and mitochondrial transcripts in BBB at 24 h after AKI. PGC-1α transfection improved renal function, systemic inflammation and BBB permeability via both the paracellular and transcellular pathways. Further study suggested that PGC-1α overexpression elevated fatty acid oxidation related gene expression. Our findings demonstrate the importance of PGC-1α in AKI-induced BBB injury and suggest that it could be a therapeutic target for BBB repair via the regulation of mitochondrial function.






Similar content being viewed by others
References
Doi K, Rabb H (2016) Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int 89:555–564.
Wu VC, Wu PC, Wu CH et al (2014) The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 3:e000933
Lu R, Kiernan MC, Murray A et al (2015) Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol 11:707–719
Liu M, Liang Y, Chigurupati S et al (2008) Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19:1360–1370
Doll DN, Hu H, Sun J et al (2015) Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke 46:1681–1689
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 6:361–370
Portilla D, Dai G, McClure T et al (2002) Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int 62:1208–1218
Tran M, Tam D, Bardia A et al (2011) PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Investig 121:4003–4014
Tran MT, Zsengeller ZK, Berg AH et al (2016) PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531:528–532
Ruan W, Li J, Xu Y et al (2019) MALAT1 up-regulator polydatin protects brain microvascular integrity and ameliorates stroke through C/EBP/MALAT1/CREB/PGC-1α/PPARγ pathway. Cell Mol Neurobiol 39:265–286
Yang L, Brooks CR, Xiao S et al (2015) KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin investig 125:1620–1636
Firouzjaei MA, Haghani M, Shid Moosavi SM (2019) Renal ischemia/reperfusion induced learning and memory deficit in the rat: insights into underlying molecular and cellular mechanisms. Brain Res 1719:263–273
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1:409–417
Siegenthaler JA, Sohet F, Daneman R (2013) ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barrier genesis. Curr Opin Neurobiol 23:1057–1064
Knowland D, Arac A, Sekiguchi KJ et al (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82:603–617
Luissint AC, Artus C, Glacial F et al (2012) Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9:23
Nguyen HM, Mejia EM, Chang W et al (2016) Reduction in cardiolipin decreases mitochondrial spare respiratory capacity and increases glucose transport into and across human brain cerebral microvascular endothelial cells. J Neurochem 139:68–80
Ben-Zvi A, Lacoste B, Kur E et al (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509:507–511
Nguyen LN, Ma D, Shui G et al (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509:503–506
Yang YR, Xiong XY, Liu J et al (2017) Mfsd2a (major facilitator superfamily domain containing 2a) attenuates intracerebral hemorrhage-induced blood-brain barrier disruption by inhibiting vesicular transcytosis. J Am Heart Assoc 6:e005811
He YJ, Xu H, Fu YJ et al (2018) Intraperitoneal hypertension, a novel risk factor for sepsis-associated encephalopathy in sepsis mice. Sci Rep 8:8173
Tiwary S, Morales JE, Kwiatkowski SC et al (2018) Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci Rep 8:8267
Nag S (2003) Pathophysiology of blood-brain barrier breakdown. Methods Mol Med 89:97–119
Bell RD, Zlokovic BV (2009) Neurovascular mechanisms and blood brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118:103–113
Berger JH, Charron MJ, Silver DL (2012) Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS ONE 7:e50629
Nongnuch A, Panorchan K, Davenport A (2014) Brain-kidney crosstalk. Crit Care 18:225
Chen J, John R, Richardson JA et al (2011) Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int 80:504–515
Handschin C, Chin S, Li P et al (2007) Skeletal muscle fiber type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knockout animals. J Biol Chem 282:30014–30021
Handschin C, Choi CS, Chin S et al (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell crosstalk. J Clin investig 117:3463–3474
Wenz T, Rossi SG, Rotundo RL et al (2009) Increased muscle PGC-1a expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 106:20405–20410
Yao D, Kuwajima M, Chen Y et al (2007) Impaired long-chain fatty acid metabolism in mitochondria causes brain vascular invasion by a non-neurotropic epidemic influenza A virus in the newborn/suckling period: implications for influenza-associated encephalopathy. Mol Cell Biochem 299:85–92
Arany Z, Foo SY, Ma Y et al (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451:1008–1012
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81601720).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, H., Li, J., Zhou, Q. et al. Protective Effects of PGC-1α on the Blood Brain Barrier After Acute Kidney Injury. Neurochem Res 45, 1086–1096 (2020). https://doi.org/10.1007/s11064-020-02985-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02985-5