Abstract
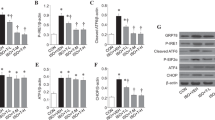
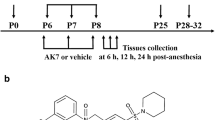
Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) has recently been shown to promote oxidative stress and inflammation in the peripheral tissues, contributing to the pathogenesis of many diseases. Here we examined whether pre-existing higher circulating TMAO would influence cognitive function in aged rats after anesthetic sevoflurane exposure. Aged rats received vehicle or TMAO treatment for 3 weeks. After 2 weeks of treatment, these animals were exposed to either control or 2.6% sevoflurane for 4 h. One week after exposure, freezing as measured by fear conditioning test, microglia activity, proinflammatory cytokine expression and NADPH oxidase-dependent reactive oxygen species (ROS) production in the hippocampus (a key brain structure involved in learning and memory) were comparable between vehicle-treated rats exposed to control and vehicle-treated rats exposed to sevoflurane. TMAO treatment, which increased plasma TMAO before and 1 week after control or sevoflurane exposure, significantly reduced freezing to contextual fear conditioning, which was associated with increases in microglia activity, proinflammatory cytokine expression and NADPH oxidase-dependent ROS production in the hippocampus in rats exposed to sevoflurane but not in rats exposed to control. Moreover, hippocampal expression of antioxidant enzyme methionine sulfoxide reductase A (MsrA) was reduced by TMAO treatment in both groups, and TMAO-induced reduction in MsrA expression was negatively correlated with increased proinflammatory cytokine expression in rats exposed to SEV. These findings suggest that pre-existing higher circulating TMAO downregulates antioxidant enzyme MsrA in the hippocampus, which may sensitize the hippocampus to oxidative stress, resulting in microglia-mediated neuroinflammation and cognitive impairment in aged rats after sevoflurane exposure.








Similar content being viewed by others
References
Rundshagen I (2014) Postoperative cognitive dysfunction. Dtsch Arztebl Int 111:119–125
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L (2018) Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev 84:116–133
Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX, Yang JJ (2016) NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51:109–118
Lee YM, Song BC, Yeum KJ (2015) Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int 2015:242709
Wang DS, Orser BA (2011) Inhibition of learning and memory by general anesthetics. Can J Anaesth 58:167–177
Callaway JK, Jones NC, Royse AG, Royse CF (2015) Memory impairment in rats after desflurane anesthesia is age and dose dependent. J Alzheimers Dis 44:995–1005
Callaway JK, Jones NC, Royse AG, Royse CF (2012) Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology 117:1091–1101
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118:502–515
Li D, Liu L, Li L, Li X, Huang B, Zhou C, Zhang Z, Wang C, Dong P, Zhang X, Yang B, Zhang L (2017) Sevoflurane induces exaggerated and persistent cognitive decline in a type II diabetic rat model by aggregating hippocampal inflammation. Front Pharmacol 8:886
Dong P, Zhao J, Li N, Lu L, Li L, Zhang X, Yang B, Zhang L, Li D (2018) Sevoflurane exaggerates cognitive decline in a rat model of chronic intermittent hypoxia by aggravating microglia-mediated neuroinflammation via downregulation of PPAR-gamma in the hippocampus. Behav Brain Res 347:325–331
Subramaniam S, Fletcher C (2018) Trimethylamine N-oxide: breathe new life. Br J Pharmacol 175:1344–1353
Zeisel SH, Warrier M (2017) Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 37:157–181
Li T, Chen Y, Gua C, Li X (2017) Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 8:350
Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM (2016) Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc 5:e002767
Sun G, Yin Z, Liu N, Bian X, Yu R, Su X, Zhang B, Wang Y (2017) Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem Biophys Res Commun 493:964–970
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ (2016) Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail 9:e002314
Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J (2014) Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 30:1700–1705
Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, Rey FE (2018) The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimers Res Therapy 10:124
Del Rio D, Zimetti F, Caffarra P, Tassotti M, Bernini F, Brighenti F, Zini A, Zanotti I (2017) The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients 9:1053
Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF (2012) Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci 32:14641–14648
Fonken LK, Frank MG, D'Angelo HM, Heinze JD, Watkins LR, Lowry CA, Maier SF (2018) Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol Aging 71:105–114
Stratmann G, Sall JW, Bell JS, Alvi RS, May L, Ku B, Dowlatshahi M, Dai R, Bickler PE, Russell I, Lee MT, Hrubos MW, Chiu C (2010) Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats. Anesthesiology 112:305–315
Feng X, Degos V, Koch LG, Britton SL, Zhu Y, Vacas S, Terrando N, Nelson J, Su X, Maze M (2013) Surgery results in exaggerated and persistent cognitive decline in a rat model of the metabolic syndrome. Anesthesiology 118:1098–1105
Dasgupta A, Baby N, Krishna K, Hakim M, Wong YP, Behnisch T, Soong TW, Sajikumar S (2017) Substance P induces plasticity and synaptic tagging/capture in rat hippocampal area CA2. Proc Natl Acad Sci USA 114:E8741–E8749
Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E (2010) Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res 1326:96–104
Deacon RM (2013) Measuring motor coordination in mice. J Vis Exp. https://doi.org/10.3791/2609
Wagner JM, Sichler ME, Schleicher EM, Franke TN, Irwin C, Low MJ, Beindorff N, Bouter C, Bayer TA, Bouter Y (2019) Analysis of motor function in the Tg4-42 mouse model of Alzheimer's Disease. Front Behav Neurosci 13:107
Nakamura M, Tazaki F, Nomura K, Takano T, Hashimoto M, Hashizume H, Kamei I (2017) Cognitive impairment associated with locomotive syndrome in community-dwelling elderly women in Japan. Clin Interv Aging 12:1451–1457
Jiang B, Moskovitz J (2018) The functions of the mammalian methionine sulfoxide reductase system and related diseases. Antioxidants (Basel). https://doi.org/10.3390/antiox7090122
Luo S, Levine RL (2009) Methionine in proteins defends against oxidative stress. FASEB J 23:464–472
Fan H, Wu PF, Zhang L, Hu ZL, Wang W, Guan XL, Luo H, Ni M, Yang JW, Li MX, Chen JG, Wang F (2015) Methionine sulfoxide reductase A negatively controls microglia-mediated neuroinflammation via inhibiting ROS/MAPKs/NF-kappaB signaling pathways through a catalytic antioxidant function. Antioxid Redox Signal 22:832–847
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63
Velasquez MT, Ramezani A, Manal A, Raj DS (2016) Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel) 8:326
Canyelles M, Tondo M, Cedo L, Farras M, Escola-Gil JC, Blanco-Vaca F (2018) Trimethylamine N-oxide: a link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int J Mol Sci 19:3228
Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR (1999) Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem 73:1660–1666
Sivandzade F, Bhalerao A, Cucullo L (2019) Cerebrovascular and neurological disorders: protective role of NRF2. Int J Mol Sci 20:3433
Huang L, Huang K, Ning H (2018) Hispidulin prevents sevoflurane- Induced memory dysfunction in aged rats. Biomed Pharmacother 97:412–422
Abou El-Ezz D, Maher A, Sallam N, El-Brairy A, Kenawy S (2018) Trans-cinnamaldehyde modulates hippocampal Nrf2 Factor and inhibits amyloid beta aggregation in LPS-induced neuroinflammation mouse model. Neurochem Res 43:2333–2342
Magesh S, Chen Y, Hu L (2012) Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev 32:687–726
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN (2008) Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489
Rolls ET, Kesner RP (2006) A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 79:1–48
Yang S, Yang S, Moreira T, Hoffman G, Carlson GC, Bender KJ, Alger BE, Tang CM (2014) Interlamellar CA1 network in the hippocampus. Proc Natl Acad Sci USA 111:12919–12924
Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN (2009) Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 14:959–967
Ianov L, De Both M, Chawla MK, Rani A, Kennedy AJ, Piras I, Day JJ, Siniard A, Kumar A, Sweatt JD, Barnes CA, Huentelman MJ, Foster TC (2017) Hippocampal transcriptomic profiles: subfield vulnerability to age and cognitive impairment. Front Aging Neurosci 9:383
Zhu G, Tao L, Wang R, Xue Y, Wang X, Yang S, Sun X, Gao G, Mao Z, Yang Q (2017) Endoplasmic reticulum stress mediates distinct impacts of sevoflurane on different subfields of immature hippocampus. J Neurochem 142:272–285
Acknowledgements
The present study was supported by the 960th Hospital of the PLA and PKU Care Zibo Hospital, Grant number (160013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, L., Zhang, C., Cao, G. et al. Higher Circulating Trimethylamine N-oxide Sensitizes Sevoflurane-Induced Cognitive Dysfunction in Aged Rats Probably by Downregulating Hippocampal Methionine Sulfoxide Reductase A. Neurochem Res 44, 2506–2516 (2019). https://doi.org/10.1007/s11064-019-02868-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02868-4