Abstract
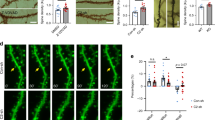
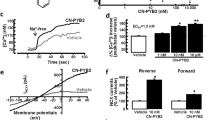
The hippocampus is critical for memory and emotion and both N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) receptors are known to contribute for those processes. However, the underlying molecular mechanisms remain poorly understood. We have previously found that mice undergo memory decline upon dcf1 deletion through ES gene knockout. In the present study, a nervous system-specific dcf1 knockout (NKO) mouse was constructed, which was found to present severely damaged neuronal morphology. The damaged neurons caused structural abnormalities in dendritic spines and decreased synaptic density. Decreases in hippocampal NMDA and AMPA receptors of NKO mice lead to abnormal long term potentiation (LTP) at DG, with significantly decreased performance in the water maze, elevated- plus maze, open field and light and dark test. Investigation into the underlying molecular mechanisms revealed that dendritic cell factor 1 (Dcf1) contributes for memory and emotion by regulating NMDA and AMPA receptors. Our results broaden the understanding of synaptic plasticity’s role in cognitive function, thereby expanding its known list of functions.




Similar content being viewed by others
References
Malenka RC, Nicoll RA (1999) Long-term potentiation—a decade of progress? Science 285(5435):1870–1874
Muller D et al (2002) LTP, memory and structural plasticity. Curr Mol Med 2(7):605–611
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129(Pt 7):1659–1673
Matsuzaki M et al (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–766
Kirkwood A, Bear MF (1994) Hebbian synapses in visual cortex. J Neurosci 14(3 Pt 2):1634–1645
Myhrer T (2003) Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Brain Res Rev 41(2–3):268–287
Cormier RJ, Greenwood AC, Connor JA (2001) Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol 85(1):399–406
Baudry M et al (2015) Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res 1621:73–81
Traynelis SF et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496
Demidchik V et al (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220(1):49–69
Collingridge GL et al (2013) The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64:13–26
Zhu S, Paoletti P (2015) Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol 20:14–23
Nowak L et al (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307(5950):462–465
Henley JM, Barker EA, Glebov OO (2011) Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34(5):258–268
Wen T, Gu P, Chen F (2002) Discovery of two novel functional genes from differentiation of neural stem cells in the striatum of the fetal rat. Neurosci Lett 329(1):101–105
Wang L et al (2008) A novel function of dcf1 during the differentiation of neural stem cells in vitro. Cell Mol Neurobiol 28(6):887–894
Li X et al (2012) MicroRNA-351 regulates TMEM 59 (DCF1) expression and mediates neural stem cell morphogenesis. RNA Biol 9(3):292–301
Boada-Romero E et al (2013) TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. EMBO J 32(4):566–582
Liu Q et al (2018) Dcf1 triggers dendritic spine formation and facilitates memory acquisition. Mol Neurobiol 55(1):763–775
Wang XD et al (2011) Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci 31(38):13625–13634
Chong SA et al (2011) Synaptic dysfunction in hippocampus of transgenic mouse models of Alzheimer's disease: a multi-electrode array study. Neurobiol Dis 44(3):284–291
Balemans MC et al (2013) Hippocampal dysfunction in the Euchromatin histone methyltransferase 1 heterozygous knockout mouse model for Kleefstra syndrome. Hum Mol Genet 22(5):852–866
Pullen AH (1990) Morphometric evidence from C-synapses for phased Nissl body response in alpha-motoneurones retrogradely intoxicated with diphtheria toxin. Brain Res 509(1):8–16
Niu J et al (2015) Propidium iodide (PI) stains Nissl bodies and may serve as a quick marker for total neuronal cell count. Acta Histochem 117(2):182–187
Banerjee J, Fischer CC, Wedegaertner PB (2009) The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry 48(33):8032–8043
Tschida KA, Mooney R (2012) Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73(5):1028–1039
Gipson CD, Olive MF (2017) Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav 16(1):101–117
Acknowledgements
This work was supported by the National Science Foundation of China (Grant Nos. 81271253, and 81471162), the Science and Technology Commission of Shanghai (Grant No. 14JC1402400), and the Key Innovation Project of Shanghai Municipal Education Commission (Grant No. 14ZZ090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, Q., Xie, J. et al. Dcf1 Affects Memory and Anxiety by Regulating NMDA and AMPA Receptors. Neurochem Res 44, 2499–2505 (2019). https://doi.org/10.1007/s11064-019-02866-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02866-6