Abstract
Luteolin, a flavonoid isolated from Cirsium japonicum, has antioxidant, anti-inflammatory and neuroprotective activities. Our previous studies brought a prospect that luteolin benefited diabetic rats with cognitive impairments. In this study, we examined whether luteolin could suppress the inflammatory cytokines, thus increasing synapse-associated proteins in streptozotocin (STZ)-induced diabetes in rat models. The model rats underwent luteolin treatment for 8 consecutive weeks, followed by assessment of cognitive performances with MWM test. Nissl staining was employed to assess the neuropathological changes in the hippocampus and the effects of luteolin on diabetic rats. With animals sacrificed, expressions of inflammatory cytokines including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) and synapse-associated proteins including growth-associated protein-43 (GAP-43) and synaptophysin (SYN) were determined. The results affirmed improvement of behavioral performances in the MWM test, downexpression of glycation end products (AGEs) in the plasma and the receptor for advanced glycation end products in the hippocampus, inhibition of IL-1β and TNF-α in both the hippocampus and plasma in diabetic rats. Furthermore, luteolin treatment upregulated the expressions of GAP-43 and SYN in the hippocampus. Thus, luteolin could ameliorate the cognitive dysfunctions in STZ-induced diabetic rat model.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) has become a major health threat worldwide, with ample evidence indicating DM as an increased risk of cognitive decline and dysfunction [1,2,3]. DM patients often exhibit reduced psychomotor efficiency [4], cognitive flexibility and rapid information-processing dysfunctions [5]. Moreover, the impact of hyperglycemia on brain and its functionalities is evidently illustrated in Alzheimer-type neuropathological changes among diabetes individuals [6]. Additionally, spatial learning impairment is reportedly associated with the impairment of central nervous system in STZ-induced diabetic rat model [7, 8].
In DM, excessive advanced glycation end products (AGEs) are accumulated [9]. The receptor for advanced glycation end products (RAGE) belongs to the superfamily of immunoglobulins and functions as a central signal transduction receptor of AGEs. The interactions between excessive AGEs and RAGE can deteriorate body functions by interfering with activities of glycated macromolecules and enzymes, leading to inflammatory responses [10]. Moreover, AGEs can impair synapse and memory functionality and efficiently exacerbate memory deterioration [11]. DM reportedly results in release of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the plasma and brain tissue [12, 13], all of which contribute to the accelerated neurodegeneration [14,15,16]. The elevated levels of IL-1β and TNF-α can trigger and maintain numerous deleterious changes in the brain, with the synaptic architectures and functionalities significantly compromised [17,18,19,20]. Growth-associated Protein-43 (GAP-43) is located on the growth cones of axons and is involved in structural synaptic plasticity in the nervous system [21] and is downregulated in diabetic rats [22]. Synaptophysin (SYN) is a vesicle-associated protein which is rapidly recruited to presynaptic terminals in response to presynaptic neuronal activity [23]. SYN is downregulated in the hippocampus in rats with STZ-induced diabetes [24, 25].
Luteolin has been investigated for its antioxidant, anti-inflammatory and neuroprotective activities [26, 27]. In addition, luteolin can reportedly suppress the release of inflammatory cytokines and abate microglial activities, thereby improving the spatial memory in aged mice [28]. The findings in our prior experiment revealed that luteolin ameliorated the memory loss in diabetic rats [29]. Nevertheless, there is a paucity of information regarding the protective effect of luteolin on diabetes-associated neuronal impairment and memory deficit. Herein, we focused on investigating whether luteolin could suppress the inflammatory cytokines and thus increase synapse-associated proteins in STZ-induced diabetic rat models.
Materials and Methods
Animals and Diabetes Models
Male Sprague-Dawley rats (aged 8–10 weeks) (Branch of National Breeder Center of Rodents, Shanghai, China) were housed in polypropylene cages and maintained at 23 ± 1 °C under a 12-h light/dark cycle with ad libitum access to water and rodent chow. All experiments were performed in conformance with Chinese Council on Animal Care Guidelines and approved by the Institutional Animal Care Committee. Rats were randomized into four groups (n = 8 each): blank vehicle (control) group, STZ-induced diabetic group (DM), and luteolin DM groups (i.e. 50 mg/kg, LT50 group and 100 mg/kg, LT100 group). Diabetes mellitus was induced by intraperitoneal injection of STZ (60 mg/kg, Sigma-Aldrich Co. LLC, St. Louis, MO, USA) dissolved in the vehicle citrate buffer (pH 4.4, 0.1 M). Three days after STZ injection, rats presenting with fasting plasma glucose levels of over 11.1 mM (200 mg/dl) and signs of polyuria, polyphagia and polydipsia were recruited for the experimental protocols, with further intraperitoneal injection of STZ to induced the non-conformant rats.
Treatment Conditions
With the rat model established, luteolin (Shaanxi Sciphar Biotechnology Co., Ltd., Shanxi, China) dissolved in 0.5% (w/v) sodium carboxymethylcellulose (CMC-Na) solution was administered at daily doses of 50 mg/kg and 100 mg/kg by gavages in the corresponding diabetic rat groups till the end of eight weeks [29], in parallel with daily gavage of CMC-Na in the DM and control groups. In addition, body weight and fasting plasma glucose levels were measured prior and subsequent to the luteolin treatments. The treatment lasted for 8 weeks commencing from DM model establishment. Thereafter, animals were scored for learning and memory task in Morris water maze for 5 consecutive days. Subsequently, all animals underwent deep anesthesia and blood collection via femoral vein for serum separation. After sacrifice, brain tissues were rapidly removed and the hippocampus isolated.
Morris Water Maze Test
The Morris water maze (MWM) test was performed [30] with minor modifications. With the rat diabetic model established after STZ injection for 3 days, rats were given luteolin for 8 consecutive weeks, followed by the MWM. The test was carried out in a circular pool (diameter, 120 cm; height, 60 cm) filled with water. Poured milk into the pool to make it opaque and controlled the water temperature at 24 ± 1 °C. The pool was divided into four quadrants, in which an escape platform was hidden in the third quadrant. The rats received four consecutive daily training trials. Each trial lasted until the rats found a platform within 90 s. If a rat did not find the escape platform within 90 s, it was given a latency score of 90 s. A probe trial was performed on the fifth day, the rats were placed and released opposite the site where the platform had been located. The probe trial consisted of a 90 s free swim in the pool without the platform. The percentage of time spent in the target quadrant and number of crossing to target platform indicates the degree of memory consolidation. Thereafter, the animals underwent sodium pentobarbital (50 mg/kg, i.p.) anesthesia and blood collection from the femoral vein, followed by rat sacrifice and isolations of the rat brain and the hippocampus, which were to be stored at − 80 °C until subsequent procedures.
Nissl Staining
Subsequent to the behavioral test, rats were subjected to sodium pentobarbital anesthesia (50 mg/kg, i.p.), followed by heart exposure and transcardial perfusion with ice-cold normal saline via the left ventricle for blood clearance and tissue fixation by 4% paraformaldehyde. The brain tissues were removed and immersed in 4% paraformaldehyde for 72 h for fixation, followed by cryoprotection in 15% sucrose solution for 24 h and 30% sucrose solution for 12 h. For Nissl staining, rat brain tissues were coronally prepared at the thickness of 30 µm using frozen sectioning technique (model: E, Thermo, USA). The thick sections in each group were stained with Cresol Blue, soaked in 0.1% cresol violet staining solution, dehydrated by 95% or 100% anhydrous ethanol and washed in dimethylbenzene. The population of positive cells in the hippocampal CA1 area was microscopically counted by an investigator blinded to the protocol. All procedures were in triplicate, with the mean calculated from bilateral fields. Data were represented as cell count per mm2.
ELISA
Blood samples were centrifuged at 12,000 rpm for 10 min at 4 °C to obtain serum. The concentrations of AGEs, IL-1β and TNF-α concentration in the plasma were examined using corresponding rat enzyme-linked immunosorbent (ELISA) kits [31] (Westang Bio-tech Co. Ltd., Shanghai, China) according to the manufactures’ instructions.
Reverse Transcriptase-PCR (RT-PCR)
For mRNA quantification, total RNA was extracted with RNAqueous kit (Applied Biosystems, Foster City, CA, USA). The cDNA was synthesized using High Capacity RNA-to-cDNA kit (Applied Biosystems) as indicated by the manufactures’ instructions. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for normalization in parallel with each gene examined. Amplified products were separated by electrophoresis on a 1% agarose gel, followed by visualization under a UV trans-illuminator and photography. To verify reproducibility, each brain sample was analyzed in triplicate for each gene (IL-1β, 5′-TTGACGGACCCCAAAAGATG-3′(F); IL-1β, 5′-AGAAGGTGCTCATGTCCTCA-3′(R); TNF-α, 5′-GGGAGTAGACAAGGTACAAC-3′(R); TNF-α, 5′-TCTCATCAGTTCTATGGCCC-3′(F); GAPDH, 5′-CCTTCCGTGTTCCTACCC-3′(F); GAPDH, 5′-CAACCTGGTCCTCAGTGTAG-3′(R)). The values for the target gene expression were normalized to GADPH and quantified relative to the expression in control samples. The products were analyzed by densitometry using the Quantity One 1-D analysis software (BioRad, Hercules, CA, USA), with quantification performed relative to GADPH (Table 1).
Western Blot Analysis
For protein extraction, frozen hippocampus was homogenized in ice-cold extraction buffer (20 mM Tris–HCl buffer, pH 7.6, 150 mM NaCl, 2 mM EDTA·2Na, 50 mM sodium fluoride, 1 mM sodium vanadate, 1% Nonidet P-40, 1% sodium deoxy cholate, 0.1% SDS, 1 mg/ml aprotinin and 1 mg/ml leupeptin). Homogenates were centrifuged at 12,000 rpm for 10 min at 4 °C to obtain supernatants, which was determined for protein concentrations by a Pierce BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Equivalent proteins (20 µg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Biosharp Biotech CO. Ltd., Hefei, Anhui, China). The membrane was blocked with 5% nonfat milk in a washing buffer [Tris-buffered saline containing 0.05% (v/v) Tween 20] at room temperature (r/t) for 2 h, followed by incubation overnight with the primary antibodies (RAGE, 1:1000, #4679S, SYN, 1:1000, #6855S, and GAP-43, 1:1000, #5307S, Cell Signaling Technology, Inc., Danvers, MA, USA). With each membrane twice rinsed for 15 min, the rabbit polyclonal antibodies (Santa Cruz Biotechnology, CA, USA) against the active RAGE, SYN and GAP-43 were incubated and visualized by 3,3′,5,5′-tetramethylbenzidine-stabilized substrate (Promega, Madison, WI, USA). β-actin (1:1000; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) served as an internal control for the cytosolic extracts. The membranes were exposed to BCIP/NBT alkaline phosphatase color developing reagent (Beyotime) for 15 min. Comparison between treatment groups was performed by determination of protein/β-actin ratio of the immunoreactive area by densitometry. Protein bands were quantified by densitometry using Scion Image analysis software (Scion Corp., Frederick, MD, USA). The OD values were normalized using β-actin as an internal control.
Statistical Analysis
All statistical analyses were performed by the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean ± SEM (n = 3 each group). Statistical significance was set at P < 0.05. The intergroup differences were analyzed using one-way ANOVA followed by the Turkey’s tests.
Results
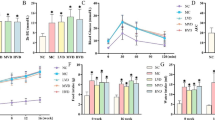
Effect of Luteolin on Body Weight and Fasting Plasma Glucose Levels in Rats
Compared with the control rats, diabetic rats exhibited significant increase in fasting plasma glucose levels (P < 0.01, Fig. 1a), as well as a marked decline in body weight (P < 0.05, P < 0.01, Fig. 1b). At the end of luteolin treatment, the fasting plasma glucose levels were significantly reduced in both treatment groups versus DM group (all P < 0.001; Fig. 1a). The body weight in LT100 group was markedly increased versus DM group (P < 0.05; Fig. 1b).
Effect of Luteolin on MWM Test in Rats
To examine whether luteolin could attenuate the diabetes-induced memory impairments, we tested the spatial learning and memory with the MWM test. At day 1, there was no significant difference in mean escape latency among groups (Fig. 2a), and at day 2, significant differences were evident in escape latency between the diabetic and the control rats (P < 0.001). From the third day, the escape latency was significantly decreased in groups LT50 (P < 0.01, P < 0.001) and LT100 (P < 0.01, P < 0.001) as compared to the DM group.
At day 5, in the probe trial of the MWM test, the rats showed a significant difference in behavioral performance. The numbers of crossings in both the arena (P < 0.05, Fig. 2b) and the time spent in target quadrant (P < 0.05, Fig. 2c) were significantly reduced in DM group versus the control group, reflecting memory impairment. The number of crossings in the arena (P < 0.05, Fig. 2b) and the time spent in the target quadrant (P < 0.05, Fig. 2c) were significantly increased with luteolin (100 mg/kg) treatment.
Nissl Staining in the Hippocampus
Following Nissl staining of the hippocampus in each group, the hippocampal CA1 subfields were observed for microphotography. The number of surviving neurons was significantly lower in the hippocampus in DM group than in the control group (P < 0.001, Fig. 3b). Administrations of luteolin (50 and 100 mg/kg) significantly increased the amount of surviving neurons versus the DM group (P < 0.01; P < 0.001).
Representative Nissl staining in the hippocampal CA1 subfield (a) The population of intact cells in the hippocampal CA1 subfield were counted as shown respectively in (b) Data are expressed as mean ± S.E.M., n = 3. ***P < 0.001 as compared to the control group; ##P < 0.01, ###P < 0.001 as compared to the DM group
Effect of Luteolin on the Expression of AGEs and RAGE in Diabetic Rats
AGEs levels were significantly increased in the serum (P < 0.001; Fig. 4a) in DM group versus the control group. However, luteolin treatment (50 and 100 mg/kg) resulted in significant decrease in the levels of AGEs (P < 0.01; P < 0.001) versus the DM group.
Diabetic rats exhibited significantly increased RAGE expression versus the control rats (P < 0.05, Fig. 4c) in the hippocampus. Also, there was a marked decline in the RAGE expression of LT100 group versus DM group (P < 0.05).
Effect of Luteolin on Inflammatory Response in the Hippocampus and Serum
The expressions of IL-1β and TNF-α mRNA in the hippocampus were determined to assess the effects of luteolin on the levels of inflammatory cytokines. As shown in Fig. 5, the expressions of IL-1β (P < 0.05, Fig. 5b) and TNF-α (P < 0.05, Fig. 5d) mRNA were significantly upregulated in the hippocampus in diabetic group versus the control group. Treatments with luteolin (50 and 100 mg/kg) significantly downregulated the TNF-α mRNA expression in hippocampus (P < 0.05; P < 0.01; Fig. 5d) in diabetic rats versus DM rats. In addition, treatment with luteolin (100 mg/kg) significantly downregulated the IL-1β mRNA expression in hippocampus (P < 0.05; Fig. 5b) in diabetic rats versus DM group.
DM is characterized by excessive peripheral inflammation. Thus, we investigated whether luteolin could have any effect on the expressions of serum IL-1β and TNF-α in diabetic rats. By ELISA, expressions of IL-1β (P < 0.05, Fig. 6a) and TNF-α (P < 0.01, Fig. 6b) were significantly upregulated in diabetic rat serum versus the control group. Luteolin administrations (50 and 100 mg/kg) significantly downregulated the TNF-α expression (P < 0.05; P < 0.01; Fig. 6b) and the IL-1β expression in LT100 group (P < 0.05; Fig. 6a) was significantly downregulated, with no significant difference in LT50 group (P > 0.05; Fig. 6a) versus the DM rats.
Effect of Luteolin on the Expression of Synapse-Associated Proteins
To further determine the neuroprotective effect of luteolin, we assessed variations of two representative synapse-associated proteins, GAP-43 and SYN, in the luteolin-treated diabetic rats.
Western blotting analysis showed that GAP-43 (P < 0.01, Fig. 7b) and SYN (P < 0.05, Fig. 7c) levels in hippocampus in the diabetic rats were significantly decreased versus control rats. Treatments with luteolin (50 and 100 mg/kg) significantly upregulated the GAP-43 expression in the hippocampus (P < 0.05; P < 0.05; Fig. 7b) in the diabetic rats versus DM group, and the SYN expression in the hippocampus was significantly upregulated with luteolin treatment (100 mg/kg) (P < 0.05; Fig. 7c) versus the DM group.
Discussion
DM has become a serious health risk worldwide. In the present study experiment with rat model of DM, we demonstrated that luteolin could not only attenuate inflammatory responses by downregulating AGEs and RAGE, but also improve cognitive behaviors by upregulating the synapse-associated proteins in the hippocampus in STZ-induced diabetic rat model.
In this study, we affirmed that the diabetic rats exhibited increased escape latency and the abridged duration in target quadrant in Morris water maze test, indicating impairment to the abilities of spatial learning and memory. We also demonstrated that long-term treatment with luteolin at high doses significantly reversed these behavioral changes, validating the protective potential of luteolin against diabetes-induced cognitive impairment. Our assessments of blood glucose and body weight in each group revealed that long-term luteolin treatment at both low and high doses significantly decreased blood glucose in the STZ-induced diabetic rats. Luteolin at high dose also increased body weight in STZ-induced rat model.
Increasing evidence shows that oxidative stress under diabetes mellitus can result in the excessive activation of reactive oxygen species (ROS), and the elevated of ROS may affect the downstream RAGE/AGE pathway [32]. Activate RAGE/AGE pathway increases inflammation factor, leading to the production of ROS. In addition, the elevated ROS could activate the inflammatory signal, further to promote the expression of inflammatory mediators such as TNF-α and IL-6 [33, 34]. Furthermore, upregulating the level of inflammatory factor IL-1β may damage cognitive function [35, 36]. Based on our previous study [29], STZ induced neuronal damage, cognitive dysfunction and aggravated oxidative stress. Luteolin could improve neuronal damage and cognitive function, and alleviate oxidative stress in diabetic rats.
Diabetes hyperglycemia reportedly enhances the production of AGEs [37,38,39] and RAGE [40], with AGEs being important pathogenetic mediators of almost all diabetic complications, including diabetic encephalopathy [41]. In our present study, the levels of AGEs in serum and the RAGE levels in hippocampus were significantly increased in diabetic rats, whereas luteolin administrations markedly decreased the AGEs and the RAGE levels, which was also supported by previous reports [42, 43]. Increased IL-1β and TNF-α expression in the serum and the hippocampus in the STZ-induced DM rats. The luteolin treatment significantly inhibited the IL-1β and TNF-α expression in the serum and the hippocampus in diabetic rats, thereby we postulated that luteolin could benefit diabetic rats via inhibition of the chronic STZ-induced inflammatory response and amelioration of cognitive impairment. Nissl staining of the hippocampus CA1 in the diabetic rats also confirmed that luteolin could evidently increase the CA1 neuron population.
GAP-43 is a crucial in axonal elongation, synaptic plasticity and nerve sprouting during neuronal development and maturation in adult rats [44, 45]. SYN levels are reportedly reduced in type 2 diabetic patients compared with control individuals [46]. Herein, we demonstrated that the levels of proteins GAP-43 and SYN in the hippocampus were decreased in the diabetic rats. More importantly, we validated that luteolin treatment significantly reversed the STZ-induced decline in both GAP-43 and SYN levels. The potential of luteolin in improving cognition may be associated with its ability to increase GAP-43 and SYN protein levels in the hippocampus in the STZ-induced rat model.
In summary, we demonstrated that luteolin exerts protective effects against the cognitive decline in rat model of STZ-induced diabetes mellitus. Luteoin might inhibit inflammatory responses either directly via the suppression of inflammatory cytokines or indirectly via the downregulation of AGEs/RAGE in STZ-induced diabetic rats, and ultimately the upregulation of synapse-associated proteins in the rat hippocampus. These behavioral and biochemical improvements may shed light on novel regimens with luteolin for diabetes-induced cognition decline.
References
Koekkoek PS, Kappelle LJ, Berg EVD, Rutten GEHM, Biessels GJ (2015) Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol 14(3):329–340
Ryo S, Ferris HA, Chee MJ, Eleftheria MF, C Ronald K (2013) Reduction of the cholesterol sensor SCAP in the brains of mice causes impaired synaptic transmission and altered cognitive function. PLoS Biol 11(4):e1001532
Qiu C, Sigurdsson S, Zhang Q, Jonsdottir MK, Kjartansson O, Eiriksdottir G, Garcia ME, Harris TB, Buchem MA, Gudnason V (2014) Diabetes, markers of brain pathology and cognitive function. Ann Neurol 75(1):138–146
Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, Dahms W, Group AE (2011) Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 54(2):245–255
Cukierman T, Gerstein HC, Williamson JD (2005) Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 48(12):2460–2469
Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M(2010) Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 75(13):1195–1202
Kalalian-Moghaddam H, Baluchnejadmojarad T, Roghani M, Goshadrou F, Ronaghi A (2012) Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur J Pharmacol 698(1–3):259–266
Zhao CH, Liu HQ, Cao R, Ji AL, Zhang L, Wang F, Yang RH (2012) Effects of dietary fish oil on learning function and apoptosis of hippocampal pyramidal neurons in streptozotocin-diabetic rats. Brain Res 1457(2):33–43
Critchley JA, Restrepo BI, Ronacher K, Kapur A, Bremer AA, Schlesinger LS, Basaraba R, Kornfeld H, van Crevel R (2017) Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 2: underlying biological mechanisms. Chest 152:165–173
Stirban A, Gawlowski T, Roden M (2014) Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol Metab 3(2):94–108
Li XH, Lv BL, Xie JZ, Jing L, Zhou XW, Wang JZ (2012) AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging 33(7):1400–1410
Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA (2009) Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab 296(5):E1164–E1171
Pancani T, Anderson KL, Brewer LD, Kadish I, Demoll C, Landfield PW, Blalock EM, Porter NM, Thibault O (2013) Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol Aging 34(8):1977–1987
Sama MA, Mathis DM, Furman JL, Hafiz Mohmmad A, Artiushin IA, Kraner SD, Norris CM (2008) Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem 283(32):21953–21964
Hennessy E, Griffin ÉW, Cunningham C (2015) Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1 and TNF. J Neurosci 35(22):8411–8422
Alexander K, Clarissa VH, Marco S, Annalena T, Nadine P, Mariya K, Aarne F, Spies CD (2013) Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS ONE 8(5):e62679–e62679
Barnum CJ, Tansey MG (2011) The duality of TNF signaling outcomes in the brain: potential mechanisms? Exp Neurol 229(2):198–200
Mrak RE, Griffin WS (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 26(3):349–354
Pickering M, Cumiskey D, O’Connor JJ (2005) Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol 90(5):663–670
Lynch MA (1999) What is the biological significance of an age-related increase in IL-1beta in hippocampus? Mol Psychiatry 4(1):15–18
Grasselli G (2013) Structural plasticity of climbing fibers and the growth-associated protein GAP-43. Front Neural Circuits 7(6):25
Zhou J, Wang L, Ling S, Zhang X (2007) Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol 206(2):201–208
Basu J, Siegelbaum SA (2015) The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb Perspect Biol 7(11):a021733
Hou Y, Zhou L, Yang QD, Du XP, Li M, Yuan M, Zhou ZW (2012) Changes in hippocampal synapses and learning-memory abilities in a streptozotocin-treated rat model and intervention by using fasudil hydrochloride. Neuroscience 200(1):120–129
Li C, Liu S, Guan Y, Qian W, Du F, Hou X (2009) Long pulse gastric electrical stimulation induces regeneration of myenteric plexus synaptic vesicles in diabetic rats. Neurogastroenterol Motil 22(4):453–461
Saebyeol J, Kelley KW, Johnson RW (2008) Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 105(21):7534–7539
Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T (2012) Correction: Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflamm 9(1):72–79
Jang S, Dilger RN, Johnson RW (2010) Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice 1, 2, 3. J Nutr 140(10):1892–1898
Liu Y, Tian X, Gou L, Sun L, Xin L, Yin X (2013) Luteolin attenuates diabetes-associated cognitive decline in rats. Brain Res Bull 94(9):23–29
Zheng S, Han F, Shi Y, Wen L, Han D (2017) Single-prolonged-stress-induced changes in autophagy-related proteins beclin-1, LC3, and p62 in the medial prefrontal cortex of rats with post-traumatic stress disorder. J Mol Neurosci 62:1–12
Meng Z, Zhang Y, Wei Z, Liu P, Kang J, Zhang Y, Ma D, Ke C, Chen Y, Luo J (2017) High serum resistin associates with intrahepatic inflammation and necrosis: an index of disease severity for patients with chronic HBV infection. BMC Gastroenterol 17(1):6
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le YZ, Ge J, Johnson RS, Ma JX (2011) Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia 54(6):1554–1566
Pickering RJ, Rosado CJ, Sharma A, Buksh S, Tate M, De JH (2018) Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunol 7(4):e1016
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9):732–741
刘灿: 老年抑郁患者认知功能与IL-1β水平相关性研究. 中华医学研究杂志 (2012)
Kulkarni MJ, Korwar AM, Mary S, Bhonsle HS, Giri AP (2013) Glycated proteome: from reaction to intervention. Proteom Clin Appl 7(1–2):155–170
Šebeková K, Stürmer M, Fazeli G, Bahner U, Stäb F, Heidland A (2015) Is vitamin D deficiency related to accumulation of advanced glycation end products, markers of inflammation, and oxidative stress in diabetic subjects? Biomed Res Int. https://doi.org/10.1155/2015/958097
Makita Z, Vlassara H, Cerami A, Bucala R (1992) Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem 267(8):5133–5138
Santilli F, Vazzana N, Bucciarelli LG, Davì G (2009) Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem 16(8):940–952
Muhammad S, Bierhaus A, Schwaninger M (2009) Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer’s disease. J Alzheimers Dis 16(4):775–785
Manuela A, Raffaella M, Claudio M, Francesca R, Catalano MG, Nicoletta P, Oliviero D, Giuseppe B (2005) Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology 146(12):5561–5567
Wang SH, Sun ZY (2009) Diabetes impairs hippocampal function via advanced glycation end product mediated new neuron generation in animals with diabetes-related depression. Toxicol Sci 111(1):72–79
Coggins PJ, Zwiers H (1991) B-50 (GAP-43): biochemistry and functional neurochemistry of a neuron-specific phosphoprotein. J Neurochem 56(4):1095–1106
Hung CC, Lin CH, Chang H, Wang CY, Lin SH, Hsu PC, Sun YY, Lin TN, Shie FS, Kao LS (2016) Astrocytic GAP43 induced by the TLR4/NF-κB/STAT3 axis attenuates astrogliosis-mediated microglial activation and neurotoxicity. J Neurosci 36(6):2027–2043
Claes-Goran O, Herbert G, Laura S, Annika T, Tamas B (2006) Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes 55(2):435–440
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81671069), the Open Program of Key Laboratory of Nuclear Medicine, Ministry of Health and Jiangsu Key Laboratory of Molecular Nuclear Medicine (KF201503), Xuzhou innovation of science and technology special project (No.KC16SW164, XZZDY1404), “Six-Talents Summit” Project of Jiangsu Province (2011-YY-13), and the Graduate Student Innovation Plan of Jiangsu Province (2015YKYCX015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Jx., Cheng, Xj., Luo, X. et al. Luteolin Ameliorates Cognitive Impairments by Suppressing the Expression of Inflammatory Cytokines and Enhancing Synapse-Associated Proteins GAP-43 and SYN Levels in Streptozotocin-Induced Diabetic Rats. Neurochem Res 43, 1905–1913 (2018). https://doi.org/10.1007/s11064-018-2608-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2608-6