Abstract
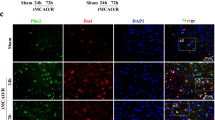
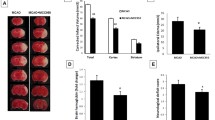
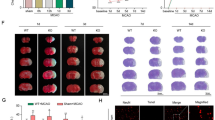
Necroptosis is a manner of caspase-independent cell death,which accounts for delayed ischemic cerebral injury, and can be used as a novel tool to expand the treatment time window in ischemic cerebral injury. Q-VD-OPH, a novel pan caspase inhibitor, has been identified as an inducer of necroptosis. In this study, we determined the optimal dose of Q-VD-OPH, which induces necroptosis in rats by the middle cerebral artery occlusion, followed by reperfusion. Furthermore, we report that the NLRP3 inflammasome is involved in necroptosis, with levels of NLRP3 inflammasome proteins as well as inflammatory cytokines, such as IL-1β, being elevated. We also demonstrated that NLRP3 was not only expressed in microglia and vascular endothelial cell, but also in neurons when necroptosis is induced with Q-VD-OPH. Inhibition of NLRP3 by glyburide strongly suppressed the expression of NLRP3 inflammasome proteins and IL-1β, and markedly reduced brain tissue damage. Our findings provide evidence that pretreatment with Q-VD-OPH suppresses apoptosis and induces necroptosis in the cerebral ischemia-reperfusion model. We also identified that the NLRP3 inflammasome plays an important role in neuronal necroptosis, and that NLRP3 inflammasome deficiency reduces brain tissue damage after cerebral ischemia-reperfusion injury in rats.




Similar content being viewed by others
References
Weber K, Schilling JD (2014) Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem 289(13):9158–9171. https://doi.org/10.1074/jbc.M113.531202
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119. https://doi.org/10.1038/nchembio711
Yang R, Hu K, Chen J, Zhu S, Li L, Lu H, Li P, Dong R (2017) Necrostatin-1 protects hippocampal neurons against ischemia/reperfusion injury via the RIP3/DAXX signaling pathway in rats. Neurosci Lett 651:207–215. https://doi.org/10.1016/j.neulet.2017.05.016
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517(7534):311–320. https://doi.org/10.1038/nature14191
Zhang L, Feng Q, Wang T (2018) Necrostatin-1 protects against paraquat-induced cardiac contractile dysfunction via RIP1-RIP3-MLKL-dependent necroptosis pathway. Cardiovasc Toxicol. https://doi.org/10.1007/s12012-017-9441-z
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66. https://doi.org/10.1016/j.brainresrev.2006.11.003
Osman AM, Neumann S, Kuhn HG, Blomgren K (2016) Caspase inhibition impaired the neural stem/progenitor cell response after cortical ischemia in mice. Oncotarget 7(3):2239–2248. https://doi.org/10.18632/oncotarget.6803
Keoni CL, Brown TL (2015) Inhibition of apoptosis and efficacy of pan caspase inhibitor, Q-VD-OPh, in models of human disease. J Cell Death 8:1–7. https://doi.org/10.4137/JCD.S23844
Moujalled DM, Cook WD, Murphy JM, Vaux DL (2014) Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis 5:e1086. https://doi.org/10.1038/cddis.2014.18
Antar V, Akdemir O, Sağmanligil A, Sahan E, Çelik Ö, Çolak A, Karaoğlan A (2009) Q-VD-OPh, a pancaspase inhibitor, reduces trauma-induced apoptosis and improves the recovery of hind-limb function in rats after spinal cord injury. Neurocirugia 20(6):533–540 (discussion 540)
Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD (2011) Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 42(4):1090–1096. https://doi.org/10.1161/STROKEAHA.110.594861
Han W, Sun Y, Wang X, Zhu C, Blomgren K (2014) Delayed, long-term administration of the caspase inhibitor Q-VD-OPh reduced brain injury induced by neonatal hypoxia-ischemia. Dev Neurosci 36(1):64–72. https://doi.org/10.1159/000357939
Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C (2007) Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem 100(4):1062–1071. https://doi.org/10.1111/j.1471-4159.2006.04269.x
Thakkar R, Wang R, Sareddy G, Wang J, Thiruvaiyaru D, Vadlamudi R, Zhang Q, Brann D (2016) NLRP3 inflammasome activation in the brain after global cerebral ischemia and regulation by 17 beta-estradiol. Oxid Med Cell Longev. https://doi.org/10.1155/2016/8309031
Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4:e790. https://doi.org/10.1038/cddis.2013.326
Takahashi M (2014) Regarding article, “NLRP3 inflammasome as a therapeutic target in myocardial infarction” reply. Int Heart J 55(4):380–380
Engel O, Kolodziej S, Dirnagl U, Prinz V (2011) Modeling stroke in mice—middle cerebral artery occlusion with the filament model. J Visual Experim. https://doi.org/10.3791/2423
Xu Y, Wang J, Song X, Qu L, Wei R, He F, Wang K, Luo B (2016) RIP3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via AIF. Sci Rep. https://doi.org/10.1038/srep29362
Hou X, Yang C, Zhang L, Hu T, Sun D, Cao H, Yang F, Guo G, Gong C, Zhang X, Tong A (2017) Killing colon cancer cells through PCD pathways by a novel hyaluronic acid-modified shell-core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials 124:195–210. https://doi.org/10.1016/j.biomaterials.2016.12.032
Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16(6):329–344. https://doi.org/10.1038/nrm3999
Mohagheghi F, Ahmadiani A, Rahmani B, Moradi F, Romond N, Khalaj L (2013) Gemfibrozil pretreatment resulted in a sexually dimorphic outcome in the rat models of global cerebral ischemia-reperfusion via modulation of mitochondrial pro-survival and apoptotic cell death factors as well as MAPKs. J Mol Neurosci 50(3):379–393. https://doi.org/10.1007/s12031-012-9932-0
Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL (2003) Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8(4):345–352
Kuželová K, Grebeňová D, Brodská B (2011) Dose-dependent effects of the caspase inhibitor Q-VD-OPh on different apoptosis-related processes. J Cell Biochem 112(11):3334–3342. https://doi.org/10.1002/jcb.23263
Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H (2012) Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36(2):215–227. https://doi.org/10.1016/j.immuni.2012.01.012
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18(10):3659–3668
D’amelio M, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17(7):1104–1114. https://doi.org/10.1038/cdd.2009.180
Haylor JL, Harris KP, Nicholson ML, Waller HL, Huang Q, Yang B (2011) Atorvastatin improving renal ischemia reperfusion injury via direct inhibition of active caspase-3 in rats. Experim Biol Med 236(6):755–763. https://doi.org/10.1258/ebm.2011.010350
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312–1316. https://doi.org/10.1126/science.281.5381.1312
Deng H, Zuo X, Zhang J, Liu X, Liu L, Xu Q, Wu Z, Ji A (2015) Alphalipoic acid protects against cerebral ischemia/reperfusion-induced injury in rats. Mol Med Rep 11(5):3659–3665. https://doi.org/10.3892/mmr.2015.3170
Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C (2007) Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem 100(4):1062–1071
Arora D, Sharma PK, Siddiqui MH, Shukla Y (2017) Necroptosis: Modules and molecular switches with therapeutic implications. Biochimie 137:35–45. https://doi.org/10.1016/j.biochi.2017.02.015
Han J, Zhong CQ, Zhang DW (2011) Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol 12(12):1143–1149. https://doi.org/10.1038/ni.2159
Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q, Wang K, Luo BY (2011) Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Experim Neurol 70(4):314–322. https://doi.org/10.1097/NEN.0b013e31821352bd
Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau-Sepulveda MV, Peterson ED, Fonarow GC (2013) Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ-Cardiovasc Qual 6(5):543–549
Allahtavakoli M, Amin F, Esmaeeli-Nadimi A, Shamsizadeh A, Kazemi-Arababadi M, Kennedy D (2015) Ascorbic acid reduces the adverse effects of delayed administration of tissue plasminogen activator in a rat stroke model. Basic Clin Pharmacol 117(5):335–339
Garcia-Bermejo P, Calleja AI, Perez-Fernandez S, Cortijo E, del Monte JM, Garcia-Porrero M, Fe Muñoz M, Fernández-Herranz R, Arenillas JF (2012) Perfusion computed tomography-guided intravenous thrombolysis for acute ischemic stroke beyond 4.5 hours: a case-control study. Cerebrovasc Dis 34(1):31–37
Adameova A, Hrdlicka J, Szobi A, Farkasova V, Kopaskova K, Murarikova M, Neckar J, Kolar F, Ravingerova T, Dhalla NS (2017) Evidence of necroptosis in hearts subjected to various forms of ischemic insults. Can J Physiol Pharmacol. https://doi.org/10.1139/cjpp-2016-0609
Saleh D, Najjar M, Zelic M, Shah S, Nogusa S, Polykratis A, Paczosa MK, Gough PJ, Bertin J, Whalen M, Fitzgerald KA (2017) Kinase activities of RIPK1 and RIPK3 can direct IFN-beta synthesis induced by lipopolysaccharide. J Immunol 198(11):4435–4447. https://doi.org/10.4049/jimmunol.1601717
Oliva-Martin MJ, Sanchez-Abarca LI, Rodhe J, Carrillo-Jimenez A, Vlachos P, Herrera AJ, Garcia-Quintanilla A, Caballero-Velazquez T, Perez-Simon JA, Joseph B, Venero JL (2016) Caspase-8 inhibition represses initial human monocyte activation in septic shock model. Oncotarget 7(25):37456–37470. https://doi.org/10.18632/oncotarget.9648
Lambertsen KL, Biber K, Finsen B (2012) Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32(9):1677–1698. https://doi.org/10.1038/jcbfm.2012.88
Fu Y, Liu Q, Anrather J, Shi FD (2015) Immune interventions in stroke. Nat Rev Neurol 11(9):524–535. https://doi.org/10.1038/nrneurol.2015.144
Seno K, Sase S, Ozeki A, Takahashi H, Ohkuchi A, Suzuki H, Matsubara S, Iwata H, Kuwayama T, Shirasuna K (2017) Advanced glycation end products regulate interleukin-1beta production in human placenta. J Reprod Dev. https://doi.org/10.1262/jrd.2017-032
Savage CD, Lopez-Castejon G, Denes A, Brough D (2012) NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. https://doi.org/10.3389/Fimmu.2012.00288
Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P, Dostert C (2015) NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PloS ONE 10(6):e0130624. https://doi.org/10.1371/journal.pone.0130624
Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 6:262. https://doi.org/10.3389/fphar.2015.00262
Won JH, Park S, Hong S, Son S, Yu JW (2015) Rotenone-induced Impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J Biol Chem 290(45):27425–27437. https://doi.org/10.1074/jbc.M115.667063
Lee HI, Lee SW, Kim NG, Park KJ, Choi BT, Shin YI, Shin HK (2017) Low-level light emitting diode (LED) therapy suppresses inflammasome-mediated brain damage in experimental ischemic stroke. J Biophotonics. https://doi.org/10.1002/jbio.201600244
Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, Trichilo V (2016) ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2183026
Liu L, Dong Y, Ye M, Jin S, Yang J, Joosse ME, Sun Y, Zhang J, Lazarev M, Brant SR, Safar B (2016) The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohn’s Colitis. https://doi.org/10.1093/ecco-jcc/jjw219
Acknowledgements
This study was supported by Grants 81501138 from the National Natural Science Foundation of China and KC15SH077 from Xuzhou Science and Technology Project. We would like to thank Hongyan Dong, Ting Li and Fuxing Dong from Laboratory of Neurobiology Research Center of Xuzhou Medical University for advices and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to disclose.
Additional information
Xue Teng and Weiwei Chen are co-first authors.
Rights and permissions
About this article
Cite this article
Teng, X., Chen, W., Liu, Z. et al. NLRP3 Inflammasome Is Involved in Q-VD-OPH Induced Necroptosis Following Cerebral Ischemia-Reperfusion Injury. Neurochem Res 43, 1200–1209 (2018). https://doi.org/10.1007/s11064-018-2537-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2537-4