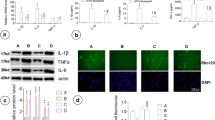
Abstract
Zn2+ plays a crucial role in the CNS where it accumulates in synaptic vesicles and is released during neurotransmission. Synaptically released Zn2+ is taken up by neurons and astrocytes. The majority of previous work has focused on neuronal damage caused by excess Zn2+. However, its effect on astrocyte function is not well understood. We examined the effect of extracellularly applied Zn2+ on nitric oxide (NO) production in primary cultured rat astrocytes, which were experimentally activated by lipopolysaccharide (LPS). Zn2+, at a concentration up to 125 μM, augmented LPS-induced NO production without affecting cell viability. LPS induced expression of both mRNA and protein of inducible NO synthase; this expression was enhanced by 125 µM Zn2+. Zn2+ also increased LPS-induced production of intracellular reactive oxygen species. Zn2+ enhanced the phosphorylation of p38-mitogen-activated protein kinase (MAPK) at 1–6 h after LPS treatment. The LPS-induced nuclear factor-kappaB (NFκB) activation was sustained for 6 h by Zn2+. Intracellular Zn2+ chelation with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) or inhibition of p38-MAPK diminished the Zn2+ enhancement of LPS-induced NO production. These findings suggest that activation of MAPK and NFκB is important for mediating Zn2+enhancement of LPS-induced NO production in astrocytes. Such changes may exacerbate glial and neuronal damage during neuroinflammation.






Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- DAN:
-
2,3-Diaminonaphthalene
- DCF:
-
Dichlorofluorescein
- DMEM:
-
Dulbecco’s modified Eagle medium
- ERK:
-
Extracellular signal-regulated kinase
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GSH:
-
Glutathione
- H2DCFDA:
-
2′,7′-dichlorodihydrofluorescein diacetate
- HBS:
-
Hepes-buffered saline
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
c-Jun N-terminal kinase
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MTT:
-
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide
- NFκB:
-
Nuclear factor-kappaB
- NO:
-
Nitric oxide
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TPEN:
-
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- ZIP:
-
Zn2+-importing protein
References
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Presad AS (1995) Zinc: an overview. Nutrition 11:93–99
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10:780–791
Sekler I, Sensi SL, Hershfinkel M, Silverman WF (2007) Mechanism and regulation of cellular zinc transport. Mol Med 13:337–343
Takeda A (2001) Zinc homeostasis and functions of zinc in the brain. Biometals 14:343–351
Frederickson CJ, Moncrieff DW (1994) Zinc-containing neurons. Biol Signals 3:127–139
Qian J, Nobels JL (2005) Visualization of transmitter releases with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol 566:747–758
Choi DW, Koh JY (1998) Zinc and brain injury. Annu Rev Neurosci 21:347–375
Vogt K, Mellor J, Tong G, Nicoll R (2000) The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26:187–196
Molnár P, Nadler JV (2001) Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Res 910:205–207
Smart TG, Hosie AM, Miller PS (2004) Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist 10:432–442
Shen Y, Yang XL (1999) Zinc modulation of AMPA receptors may be relevant to splice variants in carp retina. Neurosci Lett 259:177–180
Bishop GM, Scheiber IF, Dringen R, Robinson SR (2010) Synergistic accumulation of iron and zinc by cultured astrocytes. J Neural Transm 117:809–817
Varea E, Alonso-Llosà G, Molowny A, Lopez-Garcia C, Ponsoda X (2006) Capture of extracellular zinc ions by astrocytes. Glia 54:304–315
Segawa S, Tatsumi N, Ohishi A, Nishida K, Nagasawa K (2015) Characterization of zinc uptake by mouse primary cultured astrocytes and microglia. Metallomics 7:1067–1077
Canzoniero LM, Turetsky DM, Choi DW (1999) Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J Neurosci 19:RC31
Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY (1999) Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience 89:175–182
Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE (1994) Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 265:1464–1467
Bush AI, Pettingell WH Jr, Paradis MD, Tanzi RE (1994) Modulation of Aβ adhesiveness and secretase site cleavage by zinc. J Biol Chem 269:12152–12158
Noy D, Solomonov I, Sinkevich O, Arad T, Kjaer K, Sagi I (2008) Zinc-amyloid beta interactions on a millisecond time-scale stabilize non-fibrillar Alzheimer-related species. J Am Chem Soc 130:1376–1383
Danscher G, Jensen KB, Frederickson CJ, Kemp K, Andreasen A, Juhl S, Stoltenberg M, Ravid R (1997) Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J Neurosci Methods 76:53–59
Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26:207–214
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114:1953–1975
Frederickson CJ, Hernandez MD, McGinty JF (1989) Translocation of zinc may contribute to seizure-induced death of neurons. Brain Res 480:317–321
Kitamura Y, Iida Y, Abe J, Mifune M, Kasuya F, Ohta M, Igarashi K, Saito Y, Saji H (2006) Release of vesicular Zn2+ in a rat transient middle cerebral artery occlusion model. Brain Res Bull 69:622–625
Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ (2000) Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res 852:268–273
Rossi D, Volterra A (2009) Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull 80:224–232
Nomura Y (2001) NF-ïκB activation and IkBα dynamism involved in iNOS and chemokine induction in astroglial cells. Life Sci 68:1695–1701
Saha RN, Pahan K (2006) Signals for the induction of nitric oxide synthase in astrocytes. Neurochem Int 49:154–163
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171. doi: https://doi.org/10.1172/JCI58644
Boje KM (2004) Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci 9:763–776
Calabrese V, Mamcuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature Rev Neurosci 8:755–766
Guix FX, Uribesalgo I, Coma M, Muñoz FJ (2005) The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76:126–152
Pannu R, Singh I (2006) Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 49:170–182
Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM (2014) Inflammation in neurodegenerative diseases-an update. Immunology 142:151–166
Drouin-Ouellet J, Cicchetti F (2012) Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol Sci 33:542–551
Murakami K, Nakamura Y, Yoneda Y (2003) Potentiation by ATP of lipopolysaccharide-stimulated nitric oxide production in cultured astrocytes. Neuroscience 117:37–42
Freyer D, Weih M, Weber JR, Bürger W, Scholz P, Manz R, Ziegenhorn A, Angestwurm K, Dirnagl U (1996) Pneumococcal cell wall components induce nitric oxide synthase and TNF-alpha in astroglial-enriched cultures. Glia 16:1–6
Castano A, Herrera AJ, Cano J, Machado A (1998) Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 70:1584–1592
Nakamura Y, Kitagawa T, Ihara H, Kozaki S, Moriyama M, Kannan Y (2006) Potentiation by high potassium of lipopolysaccharide-induced nitric oxide production from cultured astrocytes. Neurochem Int 48:43–49
Moriyama M, Kurebayashi R, Kawabe K, Takano K, Nakamura Y (2016) Acetate attenuates lipopolysaccharide-induced nitric oxide production through an anti-oxidative mechanism in cultured primary rat astrocytes. Neurochem Res 41:3138–3146
Takano K, Tanaka N, Kawabe K, Moriyama M, Nakamura Y (2013) Extracellular superoxide dismutase induced by dopamine in cultured astrocytes. Neurochem Res 38:32–41
Takano K, Sugita K, Moriyama M, Hashida K, Hibino S, Choshi T, Murakami R, Yamada M, Suzuki H, Hori O, Nakamura Y (2011) A dibenzoylmethane derivative protects against hydrogen peroxide-induced cell death and inhibits lipopolysaccharide-induced nitric oxide production in cultured rat astrocytes. J Neurosci Res 89:955–965
Kleineke JW, Brand IA (1997) Rapid changes in intracellular Zn2+ in rat hepatocytes. J Pharmacol Toxicol Methods 38:181–187
Lee SY, Son DJ, Lee YK, Lee JW, Lee HJ, Yun YW, Ha TY, Hong JT (2006) Inhibitory effect of sesaminol glucosides on lipopolysaccharide-induced NF-ïκB activation and target gene expression in cultured rat astrocytes. Neurosci Res 56:204–212
Pawate S, Shen Q, Fan F, Bhat NR (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77:540–551
Bhat NR, Feinstein DL, Shen Q, Bhat AN (2002) p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem 277:29584–29592
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFïκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. doi: https://doi.org/10.1002/glia.21094
Liao SL, Ou YC, Lin SY, Kao TK, Pan HC, Chang CY, Lai CY, Lu HC, Wang WY, Chen CJ (2011) Signaling cascades mediate astrocyte death induced by zinc. Toxicol Lett 204:108–117
Paoletti P, Vergnano AM, Barbour B, Casado M (2009) Zinc at glutamatergic synapses. Neuroscience 158:126–136
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Mueller RN, Zeng Y, Balaji RV, Masalha R, Thompson RB, Fierke CA, Sarvey JM, de Valdenebro M, Prough DS, Zornow MH (2006) Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 198:285–293
Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ (2001) Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol 86:2597–2604
Bjorklund NL, Sadagoparamanujam VM, Taglialatela G (2012) Selective, quantitative measurement of releasable synaptic zinc in human autopsy hippocampal brain tissue from Alzheimer’s disease patients. J Neurosci Methods 203:146–151
Wei G, Hough CJ, Li Y, Sarvey JM (2004) Characterization of extracellular accumulation of Zn2+ during ischemia and reperfusion of hippocampus slices in rat. Neuroscience 125:867–877
Moriyama M, Jayakumar AR, Tong XY, Norenberg MD (2010) Role of mitogen-activated protein kinases in the mechanism of oxidant-induced cell swelling in cultured astrocytes. J Neurosci Res 88:2450–2458
Hsieh HL, Yang CM (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013: 484613. doi: https://doi.org/10.1155/2013/484613
Bishop GM, Dringen R, Robinson SR (2007) Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic Biol Med 42:1222–1230
Ryu R, Shin Y, Choi JW, Min W, Ryu H, Choi CR, Ko H (2002) Depletion of intracellular glutathione mediates zinc-induced cell death in rat primary astrocytes. Exp Brain Res 143:257–263
Noh KM, Koh JY (2000) Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci 20:RC111
Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor a gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641
Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26:3203–3213
Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB (2008) Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol 74:1141–1151
Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, Shibui S, Kayama T, Kitanaka C (2014) Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res 12:119–131
Liu JM, Pan F, Li L, Liu QR, Chen Y, Xiong XX, Cheng K, Yu SB, Shi Z, Yu AC, Chen XQ (2013) Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem Biophys Res Commun 437:87–93
Nuttall JR, Oteiza PI (2012) Zinc and the ERK kinases in the developing brain. Neurotox Res 21:128–141
Senftleben U, Karin M (2002) The IKK/NF-kappa B pathway. Crit Care Med 30:S18-S26
Lee J, Yim YS, Ko SJ, Kim DG, Kim CH (2011) Gap junctions contribute to astrocytic resistance against zinc toxicity. Brain Res Bull 86:314–318
Nolte C, Gore A, Sekler I, Kresse W, Hershfinkel M, Hoffmann A, Kettenmann H, Moran A (2004) ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48:145–155
Kim YH, Koh JY (2002) The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol 177:407–418
St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR (2002) Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol 282:L185-192
Frederickson CJ, Cuajungco MP, LaBuda CJ, Suh SW (2002) Nitric oxide causes apparent release of zinc from presynaptic boutons. Neuroscience 115:471–474
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Wang T, Wang CY, Shan ZY, Teng WP, Wang ZY (2012) Clioquinol reduces zinc accumulation in neuritic plaques and inhibits the amyloidogenic pathway in AβPP/PS1 transgenic mouse brain. J Alzheimers Dis 29:549–559
Johnstone JT, Morton PD, Jayakumar AR, Bracchi-Ricard V, Runko E, Liebl DJ, Norenberg MD, Bethea JR (2013) Reduced extracellular zinc levels facilitate glutamate-mediated oligodendrocyte death after trauma. J Neurosci Res 91:828–837
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Numbers 23580408 and 17K08127 (to M. M.), 15K07768 (to Y. N.), and 26850209 and 17K15390 (to K. T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moriyama, M., Fujitsuka, S., Kawabe, K. et al. Zinc Potentiates Lipopolysaccharide-induced Nitric Oxide Production in Cultured Primary Rat Astrocytes. Neurochem Res 43, 363–374 (2018). https://doi.org/10.1007/s11064-017-2431-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2431-5