Abstract
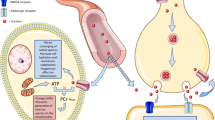
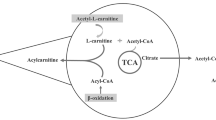
l-Carnitine functions to transport long chain fatty acyl-CoAs into the mitochondria for degradation by β-oxidation. Treatment with l-carnitine can ameliorate metabolic imbalances in many inborn errors of metabolism. In recent years there has been considerable interest in the therapeutic potential of l-carnitine and its acetylated derivative acetyl-l-carnitine (ALCAR) for neuroprotection in a number of disorders including hypoxia-ischemia, traumatic brain injury, Alzheimer’s disease and in conditions leading to central or peripheral nervous system injury. There is compelling evidence from preclinical studies that l-carnitine and ALCAR can improve energy status, decrease oxidative stress and prevent subsequent cell death in models of adult, neonatal and pediatric brain injury. ALCAR can provide an acetyl moiety that can be oxidized for energy, used as a precursor for acetylcholine, or incorporated into glutamate, glutamine and GABA, or into lipids for myelination and cell growth. Administration of ALCAR after brain injury in rat pups improved long-term functional outcomes, including memory. Additional studies are needed to better explore the potential of l-carnitine and ALCAR for protection of developing brain as there is an urgent need for therapies that can improve outcome after neonatal and pediatric brain injury.



Similar content being viewed by others
Abbreviations
- ALCAR:
-
Acetyl-l-carnitine
- OCTN2:
-
Organic cation transporter novel 2
- CPT I:
-
Carnitine palmitoyltransferase I
- CPT II:
-
Carnitine palmitoyltransferase II
- CAT:
-
Carnitine acetyltransferase
- MRS:
-
Magnetic resonance spectroscopy
- 13C-NMR:
-
13C-nuclear magnetic resonance spectroscopy
- OGD:
-
Oxygen-glucose deprivation
- HI:
-
Hypoxia-ischemia
- TBI:
-
Traumatic brain injury
- 3-NPA:
-
3-nitropropionic acid
- i.p.:
-
Intraperitoneal
- CSF:
-
Cerebrospinal fluid
- mTOR:
-
Mammalian target of rapamycin
- NGF:
-
Nerve growth factor
- pNFH:
-
Phosphorylated high-molecular weight neurofilament
- rCMRglc:
-
Cerebral regional metabolic rate of glucose
- TBI:
-
Traumatic brain injury
- TCA:
-
Tricarboxylic acid
References
Jones LL, McDonald DA, Borum PR (2010) Acylcarnitines: role in brain. Prog Lipid Res 49:61–75
Marcovina SM, Sirtori C, Peracino A, Gheorghiade M, Borum P, Remuzzi G, Ardehali H (2013) Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of l-carnitine. Transl Res 161:73–84
Ribas GS, Vargas CR, Wajner M (2014) l-Carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533:469–476
Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes Bastos M, Tavares MA, Summavielle T (2009) Acetyl-l-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience 158:514–523
Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA (2006) Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem 17:73–88
Cahova M, Chrastina P, Hansikova H, Drahota Z, Trnovska J, Skop V, Spacilova J, Malinska H, Oliyarnyk O, Papackova Z, Palenickova E, Kazdova L (2015) Carnitine supplementation alleviates lipid metabolism derangements and protects against oxidative stress in non-obese hereditary hypertriglyceridemic rats. Appl Physiol Nutr Metab 40:280–291
Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G (2005) Mechanisms of ischemic neuroprotection by acetyl-l-carnitine. Ann N Y Acad Sci 1053:153–161
Zaitone SA, Abo-Elmatty DM, Shaalan AA (2012) Acetyl-l-carnitine and alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol Biochem Behav 100:347–360
Wilson AD, Hart A, Brannstrom T, Wiberg M, Terenghi G (2007) Delayed acetyl-l-carnitine administration and its effect on sensory neuronal rescue after peripheral nerve injury. J Plast Reconstr Aesthet Surg 60:114–118
Virmani A, Koverech A, Ali SF, Binienda ZK (2011) Acetyl-l-carnitine modulates TP53 and IL10 gene expression induced by 3-NPA evoked toxicity in PC12 cells. Curr Neuropharmacol 9:195–199
Suchy J, Chan A, Shea TB (2009) Dietary supplementation with a combination of alpha-lipoic acid, acetyl-l-carnitine, glycerophosphocoline, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr Res 29:70–74
Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G (2010) Neuroprotection by acetyl-l-carnitine after traumatic injury to the immature rat brain. Dev Neurosci 32:480–487
Patel SP, Sullivan PG, Lyttle TS, Magnuson DS, Rabchevsky AG (2012) Acetyl-l-carnitine treatment following spinal cord injury improves mitochondrial function correlated with remarkable tissue sparing and functional recovery. Neuroscience 210:296–307
Kocsis K, Knapp L, Meszaros J, Kis Z, Farkas T, Vecsei L, Toldi J (2015) Acetyl-l-carnitine and oxaloacetate in post-treatment against LTP impairment in a rat ischemia model. An in vitro electrophysiological study. J Neural Transm 122:867–872
Hota KB, Hota SK, Chaurasia OP, Singh SB (2012) Acetyl-l-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus 22:723–736
Chan A, Shea TB (2007) Effects of dietary supplementation with N-acetyl cysteine, acetyl-l-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromol Med 9:264–269
Barhwal K, Hota SK, Prasad D, Singh SB, Ilavazhagan G (2008) Hypoxia-induced deactivation of NGF-mediated ERK1/2 signaling in hippocampal cells: neuroprotection by acetyl-l-carnitine. J Neurosci Res 86:2705–2721
Ishii T, Shimpo Y, Matsuoka Y, Kinoshita K (2000) Anti-apoptotic effect of acetyl-l-carnitine and I-carnitine in primary cultured neurons. Jpn J Pharmacol 83:119–124
Wainwright MS, Mannix MK, Brown J, Stumpf DA (2003) l-Carnitine reduces brain injury after hypoxia-ischemia in newborn rats. Pediatr Res 54:688–695
Wainwright MS, Kohli R, Whitington PF, Chace DH (2006) Carnitine treatment inhibits increases in cerebral carnitine esters and glutamate detected by mass spectrometry after hypoxia-ischemia in newborn rats. Stroke 37:524–530
Roe CR, Millington DS, Maltby DA, Bohan TP, Hoppel CL (1984) l-Carnitine enhances excretion of propionyl coenzyme A as propionylcarnitine in propionic acidemia. J Clin Investig 73:1785–1788
Zhang R, Zhang H, Zhang Z, Wang T, Niu J, Cui D, Xu S (2012) Neuroprotective effects of pre-treatment with l-carnitine and acetyl-l-carnitine on ischemic injury in vivo and in vitro. Int J Mol Sci 13:2078–2090
Vieira Neto E, Fonseca AA, Almeida RF, Figueiredo MP, Porto MA, Ribeiro MG (2012) Analysis of acylcarnitine profiles in umbilical cord blood and during the early neonatal period by electrospray ionization tandem mass spectrometry. Braz J Med Biol Res 45:546–556
Schmidt-Sommerfeld E, Penn D, Kerner J, Bieber LL, Rossi TM, Lebenthal E (1989) Quantitation of urinary carnitine esters in a patient with medium-chain acyl-coenzyme A dehydrogenase deficiency: effect of metabolic state and l-carnitine therapy. J Pediatr 115:577–582
Roe CR, Hoppel CL, Stacey TE, Chalmers RA, Tracey BM, Millington DS (1983) Metabolic response to carnitine in methylmalonic aciduria. An effective strategy for elimination of propionyl groups. Arch Dis Child 58:916–920
Rashed MS, Ozand PT, Bucknall MP, Little D (1995) Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res 38:324–331
Poorthuis BJ, Jille-Vlckova T, Onkenhout W (1993) Determination of acylcarnitines in urine of patients with inborn errors of metabolism using high-performance liquid chromatography after derivatization with 4′-bromophenacylbromide. Clin Chim Acta 216:53–61
Okun JG, Kolker S, Schulze A, Kohlmuller D, Olgemoller K, Lindner M, Hoffmann GF, Wanders RJ, Mayatepek E (2002) A method for quantitative acylcarnitine profiling in human skin fibroblasts using unlabelled palmitic acid: diagnosis of fatty acid oxidation disorders and differentiation between biochemical phenotypes of MCAD deficiency. Biochim Biophys Acta 1584:91–98
Novak M, Monkus EF, Chung D, Buch M (1981) Carnitine in the perinatal metabolism of lipids. I. Relationship between maternal and fetal plasma levels of carnitine and acylcarnitines. Pediatrics 67:95–100
Novak M, Monkus EF, Buch M, Silverio J, Clouston OM, Cassady JC (1988) l-Carnitine supplementation of a soybean-based formula in early infancy: plasma and urine levels of carnitine and acylcarnitines. J Pediatr Gastroenterol Nutr 7:220–224
Minkler PE, Hoppel CL (1993) Quantification of carnitine and specific acylcarnitines by high-performance liquid chromatography: application to normal human urine and urine from patients with methylmalonic aciduria, isovaleric acidemia or medium-chain acyl-CoA dehydrogenase deficiency. J Chromatogr 613:203–221
Matsumoto K, Takahashi M, Takiyama N, Misaki H, Matsuo N, Murano S, Yuki H (1993) Enzyme reactor for urinary acylcarnitines assay by reversed-phase high-performance liquid chromatography. Clin Chim Acta 216:135–143
Lloyd-Still JD, Powers CA, Wessel HU (1993) Carnitine metabolites in infants with cystic fibrosis: a prospective study. Acta Paediatr 82:145–149
Kidouchi K, Sugiyama N, Morishita H, Kobayashi M, Wada Y, Nohara D (1987) Identification of glutarylcarnitine in glutaric aciduria type 1 by carboxylic acid analyzer with an ODS reverse-phase column. Clin Chim Acta 164:261–266
Invernizzi F, Burlina AB, Donadio A, Giordano G, Taroni F, Garavaglia B (2001) Lethal neonatal presentation of carnitine palmitoyltransferase I deficiency. J Inherit Metab Dis 24:601–602
Hori T, Fukao T, Kobayashi H, Teramoto T, Takayanagi M, Hasegawa Y, Yasuno T, Yamaguchi S, Kondo N (2010) Carnitine palmitoyltransferase 2 deficiency: the time-course of blood and urinary acylcarnitine levels during initial l-carnitine supplementation. Tohoku J Exp Med 221:191–195
Xu S, Waddell J, Zhu W, Shi D, Marshall AD, McKenna MC, Gullapalli RP (2015) In vivo longitudinal proton magnetic resonance spectroscopy on neonatal hypoxic-ischemic rat brain injury: neuroprotective effects of acetyl-l-carnitine. Magn Reson Med 74:1530–1542
Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G (2016) Sex dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem 137:714–729
Tang S, Xu S, Lu X, Gullapalli RP, McKenna MC, Waddell J (2016) Neuroprotective effects of acetyl-l-carnitine on neonatal hypoxia ischemia-induced brain injury in rats. Dev Neurosci 38:384–396
Vaz FM, Wanders RJ (2002) Carnitine biosynthesis in mammals. Biochem J 361:417–429
El-Hattab AW, Scaglia F (2015) Disorders of carnitine biosynthesis and transport. Mol Genet Metab 116:107–112
Longo N, Amat di San Filippo C, Pasquali M (2006) Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C 142C:77–85
Szabo K, Nagy Z, Juhasz V, Zolnerciks JK, Csorba A, Timar Z, Molnar E, Padar P, Johnson W, Beery E, Krajcsi P (2016) Species specificity profiling of rat and human organic cation/carnitine transporter Slc22a5/SLC22A5 (Octn2/OCTN2). Drug Metab Pharmacokinet. doi:10.1016/j.dmpk.2016.08.005
Nalecz KA, Miecz D, Berezowski V, Cecchelli R (2004) Carnitine: transport and physiological functions in the brain. Mol Asp Med 25:551–567
Nalecz KA, Nalecz MJ (1996) Carnitine—a known compound, a novel function in neural cells. Acta Neurobiol Exp 56:597–609
Inazu M, Matsumiya T (2008) Physiological functions of carnitine and carnitine transporters in the central nervous system. Nihon Shinkei Seishin Yakurigaku Zasshi 28:113–120
Inazu M, Takeda H, Maehara K, Miyashita K, Tomoda A, Matsumiya T (2006) Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem 97:424–434
Miecz D, Januszewicz E, Czeredys M, Hinton BT, Berezowski V, Cecchelli R, Nalecz KA (2008) Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood–brain barrier. J Neurochem 104:113–123
Czeredys M, Mysiorek C, Kulikova N, Samluk L, Berezowski V, Cecchelli R, Nalecz KA (2008) A polarized localization of amino acid/carnitine transporter B(0,+) (ATB(0,+)) in the blood–brain barrier. Biochem Biophys Res Commun 376:267–270
Czeredys M, Samluk L, Michalec K, Tulodziecka K, Skowronek K, Nalecz KA (2013) Caveolin-1-a novel interacting partner of organic cation/carnitine transporter (Octn2): effect of protein kinase C on this interaction in rat astrocytes. PLoS ONE 8:e82105
Lamhonwah AM, Hawkins CE, Tam C, Wong J, Mai L, Tein I (2008) Expression patterns of the organic cation/carnitine transporter family in adult murine brain. Brain Dev 30:31–42
Januszewicz E, Bekisz M, Mozrzymas JW, Nalecz KA (2010) High affinity carnitine transporters from OCTN family in neural cells. Neurochem Res 35:743–748
Reuter SE, Evans AM (2012) Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet 51:553–572
Rau TF, Lu Q, Sharma S, Sun X, Leary G, Beckman ML, Hou Y, Wainwright MS, Kavanaugh M, Poulsen DJ, Black SM (2012) Oxygen glucose deprivation in rat hippocampal slice cultures results in alterations in carnitine homeostasis and mitochondrial dysfunction. PLoS ONE 7:e40881
Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, Covington JD, Bajpeyi S, Ravussin E, Kraus W, Koves TR, Mynatt RL (2012) Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15:764–777
Kawamura N (1988) Regulation of fatty acid oxidation in rat brain mitochondria: inhibition of high rates of palmitate oxidation by ADP. Arch Biochem Biophys 264:546–552
Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC (2010) Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem 114:820–831
Shinohara M, Saitoh M, Takanashi J, Yamanouchi H, Kubota M, Goto T, Kikuchi M, Shiihara T, Yamanaka G, Mizuguchi M (2011) Carnitine palmitoyl transferase II polymorphism is associated with multiple syndromes of acute encephalopathy with various infectious diseases. Brain Dev 33:512–517
Sakai E, Yamanaka G, Kawashima H, Morishima Y, Ishida Y, Oana S, Miyajima T, Shinohara M, Saitoh M, Mizuguchi M (2013) A case of recurrent acute encephalopathy with febrile convulsive status epilepticus with carnitine palmitoyltransferase II variation. Neuropediatrics 44:218–221
Celestino-Soper PB, Violante S, Crawford EL, Luo R, Lionel AC, Delaby E, Cai G, Sadikovic B, Lee K, Lo C, Gao K, Person RE, Moss TJ, German JR, Huang N, Shinawi M, Treadwell-Deering D, Szatmari P, Roberts W, Fernandez B, Schroer RJ, Stevenson RE, Buxbaum JD, Betancur C, Scherer SW, Sanders SJ, Geschwind DH, Sutcliffe JS, Hurles ME, Wanders RJ, Shaw CA, Leal SM, Cook EH Jr, Goin-Kochel RP, Vaz FM, Beaudet AL (2012) A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Natl Acad Sci USA 109:7974–7981
Rashidi-Nezhad A, Talebi S, Saebnouri H, Akrami SM, Reymond A (2014) The effect of homozygous deletion of the BBOX1 and Fibin genes on carnitine level and acyl carnitine profile. BMC Med Genet 15:75
Longo N (2016) Primary carnitine deficiency and newborn screening for disorders of the carnitine cycle. Ann Nutr Metab 68(Suppl 3):5–9
Stanley CA, Palmieri F, Bennett MJ (2014) Disorders of the mitochondrial carnitine shuttle. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson K, Mitchell G (eds) The online metabolic and molecular bases of inherited disease, McGraw-Hill, New York. http://ommbid.mhmedical.com/content.aspx?bookid=971§ionid=62633874. Accessed 20 Apr 2017
Stanley CA, Bennett MJ, Longo N (2014) Plasma membrane carnitine transporter defect, In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson K, Mitchell G (eds) The online metabolic and molecular bases of inherited disease New York, McGraw-Hill, New York. http://ommbid.mhmedical.com/content.aspx?bookid=971§ionid=62633497. Accessed 20 Apr 2017
Angelini C, Trevisan C, Isaya G, Pegolo G, Vergani L (1987) Clinical varieties of carnitine and carnitine palmitoyltransferase deficiency. Clin Biochem 20:1–7
Schuck PF, da Costa Ferreira G, Tahara EB, Klamt F, Kowaltowski AJ, Wajner M (2010) cis-4-decenoic acid provokes mitochondrial bioenergetic dysfunction in rat brain. Life Sci 87:139–146
Ferreira GC, Tonin A, Schuck PF, Viegas CM, Ceolato PC, Latini A, Perry ML, Wyse AT, Dutra-Filho CS, Wannmacher CM, Vargas CR, Wajner M (2007) Evidence for a synergistic action of glutaric and 3-hydroxyglutaric acids disturbing rat brain energy metabolism. Int J Dev Neurosci 25:391–398
Schuck PF, Milanez AP, Felisberto F, Galant LS, Machado JL, Furlanetto CB, Petronilho F, Dal-Pizzol F, Streck EL, Ferreira GC (2015) Brain and muscle redox imbalance elicited by acute ethylmalonic acid administration. PLoS ONE 10:e0126606
Spiekerkoetter U, Wood PA (2010) Mitochondrial fatty acid oxidation disorders: pathophysiological studies in mouse models. J Inherit Metab Dis 33:539–546
Stanley CA (1995) Carnitine disorders. Adv Pediatr 42:209–242
Badve MS, Bhuta S, McGill J (2015) Rare presentation of a treatable disorder: glutaric aciduria type 1. N Z Med J 128:61–64
Davies SE, Iles RA, Stacey TE, de Sousa C, Chalmers RA (1991) Carnitine therapy and metabolism in the disorders of propionyl-CoA metabolism studied using 1 H-NMR spectroscopy. Clin Chim Acta 204:263–277
Hoffmann GF, Athanassopoulos S, Burlina AB, Duran M, de Klerk JB, Lehnert W, Leonard JV, Monavari AA, Muller E, Muntau AC, Naughten ER, Plecko-Starting B, Superti-Furga A, Zschocke J, Christensen E (1996) Clinical course, early diagnosis, treatment, and prevention of disease in glutaryl-CoA dehydrogenase deficiency. Neuropediatrics 27:115–123
Kolker S, Christensen E, Leonard JV, Greenberg CR, Boneh A, Burlina AB, Burlina AP, Dixon M, Duran M, Garcia Cazorla A, Goodman SI, Koeller DM, Kyllerman M, Muhlhausen C, Muller E, Okun JG, Wilcken B, Hoffmann GF, Burgard P (2011) Diagnosis and management of glutaric aciduria type I—revised recommendations. J Inherit Metab Dis 34:677–694
Wolff JA, Carroll JE, Le Phuc T, Prodanos C, Haas R, Nyhan WL (1986) Carnitine reduces fasting ketogenesis in patients with disorders of propionate metabolism. Lancet 1:289–291
Sitta A, Vanzin CS, Biancini GB, Manfredini V, de Oliveira AB, Wayhs CA, Ribas GO, Giugliani L, Schwartz IV, Bohrer D, Garcia SC, Wajner M, Vargas CR (2011) Evidence that l-carnitine and selenium supplementation reduces oxidative stress in phenylketonuric patients. Cell Mol Neurobiol 31:429–436
Mescka CP, Wayhs CA, Vanzin CS, Biancini GB, Guerreiro G, Manfredini V, Souza C, Wajner M, Dutra-Filho CS, Vargas CR (2013) Protein and lipid damage in maple syrup urine disease patients: l-carnitine effect. Int J Dev Neurosci 31:21–24
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of l-carnitine supplementation. Int J Dev Neurosci 28:127–132
Al-sharefi A, Bilous R (2015) Reversible weakness and encephalopathy while on long-term valproate treatment due to carnitine deficiency. BMJ Case Rep doi:10.1136/bcr-2015-210727
Kim H, Chu K, Jung KH, Lee ST, Kim JM, Lee SK (2012) Acquired encephalopathy associated with carnitine deficiency after cefditoren pivoxil administration. Neurol Sci 33:1393–1396
Stanley CA (2004) Carnitine deficiency disorders in children. Ann N Y Acad Sci 1033:42–51
Clark RH, Kelleher AS, Chace DH, Spitzer AR (2014) Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics 134:e37–e46
Limketkai BN, Zucker SD (2008) Hyperammonemic encephalopathy caused by carnitine deficiency. J Gen Intern Med 23:210–213
Karakoc E, Erdem S, Sokmensuer C, Kansu T (2006) Encephalopathy due to carnitine deficiency in an adult patient with gluten enteropathy. Clin Neurol Neurosurg 108:794–797
Schols L, Zange J, Abele M, Schillings M, Skipka G, Kuntz-Hehner S, van Beekvelt MC, Colier WN, Muller K, Klockgether T, Przuntek H, Vorgerd M (2005) l-Carnitine and creatine in Friedreich’s ataxia. A randomized, placebo-controlled crossover trial. J Neural Transm 112:789–796
Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, Tanaka R, Uchiyama Y, Hattori N, Urabe T (2015) l-Carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab 35:382–391
Binienda ZK, Ali SF, Virmani A, Amato A, Salem N, Przybyla BD (2006) Co-regulation of dopamine D1 receptor and uncoupling protein-2 expression in 3-nitropropionic acid-induced neurotoxicity: neuroprotective role of l-carnitine. Neurosci Lett 410:62–65
Yu Z, Iryo Y, Matsuoka M, Igisu H, Ikeda M (1997) Suppression of pentylenetetrazol-induced seizures by carnitine in mice. Naunyn Schmiedebergs Arch Pharmacol 355:545–549
Pande SV, Blanchaer MC (1971) Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters. J Biol Chem 246:402–411
Shug AL, Shrago E, Bittar N, Folts JD, Koke JR (1975) Acyl-CoA inhibition of adenine nucleotide translocation in ischemic myocardium. Am J Physiol 228:689–692
Stumpf DA, McAfee J, Parks JK, Eguren L (1980) Propionate inhibition of succinate:CoA ligase (GDP) and the citric acid cycle in mitochondria. Pediatr Res 14:1127–1131
Matsuishi T, Stumpf DA, Seliem M, Eguren LA, Chrislip K (1991) Propionate mitochondrial toxicity in liver and skeletal muscle: acyl CoA levels. Biochem Med Metab Biol 45:244–253
Chalmers RA, Roe CR, Stacey TE, Hoppel CL (1984) Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of l-carnitine. Pediatr Res 18:1325–1328
Roe CR, Millington DS, Maltby DA, Bohan TP, Kahler SG, Chalmers RA (1985) Diagnostic and therapeutic implications of medium-chain acylcarnitines in the medium-chain acyl-coA dehydrogenase deficiency. Pediatr Res 19:459–466
Frye RE, Melnyk S, Macfabe DF (2013) Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry 3:e220
Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF (2010) Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem 113:515–529
Rosenthal RE, Williams R, Bogaert YE, Getson PR, Fiskum G (1992) Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-l-carnitine. Stroke 23:1312–1317
Virmani MA, Caso V, Spadoni A, Rossi S, Russo F, Gaetani F (2001) The action of acetyl-l-carnitine on the neurotoxicity evoked by amyloid fragments and peroxide on primary rat cortical neurones. Ann N Y Acad Sci 939:162–178
Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM (2005) Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res 79:509–521
Aureli T, Miccheli A, Di Cocco ME, Ghirardi O, Giuliani A, Ramacci MT, Conti F (1994) Effect of acetyl-l-carnitine on recovery of brain phosphorus metabolites and lactic acid level during reperfusion after cerebral ischemia in the rat—study by 13P- and 1H-NMR spectroscopy. Brain Res 643:92–99
White HL, Scates PW (1990) Acetyl-l-carnitine as a precursor of acetylcholine. Neurochem Res 15:597–601
Ricciolini R, Scalibastri M, Kelleher JK, Carminati P, Calvani M, Arduini A (1998) Role of acetyl-l-carnitine in rat brain lipogenesis: implications for polyunsaturated fatty acid biosynthesis. J Neurochem 71:2510–2517
Bogaert YE, Rosenthal RE, Fiskum G (1994) Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radic Biol Med 16:811–820
Liu Y, Rosenthal RE, Starke-Reed P, Fiskum G (1993) Inhibition of postcardiac arrest brain protein oxidation by acetyl-l-carnitine. Free Radic Biol Med 15:667–670
Chiechio S, Copani A, Nicoletti F, Gereau RW (2006) l-Acetylcarnitine: a proposed therapeutic agent for painful peripheral neuropathies. Curr Neuropharmacol 4:233–237
Janiri L, Falcone M, Persico A, Tempesta E (1991) Activity of l-carnitine and l-acetylcarnitine on cholinoceptive neocortical neurons of the rat in vivo. J Neural Transm 86:135–146
Smeland OB, Meisingset TW, Borges K, Sonnewald U (2012) Chronic acetyl-l-carnitine alters brain energy metabolism and increases noradrenaline and serotonin content in healthy mice. Neurochem Int 61:100–107
Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, Battaglia G, Mathe AA, Pittaluga A, Lionetto L, Simmaco M, Nicoletti F (2013) l-Acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 110:4804–4809
Madiraju P, Pande SV, Prentki M, Madiraju SR (2009) Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics 4:399–403
McKenna MC, Ferreira GC (2016) Enzyme complexes important for the glutamate–glutamine cycle. Adv Neurobiol 13:59–98
McKenna MC, Rae CD (2015) A new role for alpha-ketoglutarate dehydrogenase complex: regulating metabolism through post-translational modification of other enzymes. J Neurochem 134:3–6
Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S (2015) Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem 134:86–96
Kuratsune H, Watanabe Y, Yamaguti K, Jacobsson G, Takahashi M, Machii T, Onoe H, Onoe K, Matsumura K, Valind S, Kitani T, Langstrom B (1997) High uptake of [2-11C]acetyl-l-carnitine into the brain: a PET study. Biochem Biophys Res Commun 231:488–493
Aureli T, Puccetti C, Di Cocco ME, Arduini A, Ricciolini R, Scalibastri M, Manetti C, Conti F (1999) Entry of [(1,2-13C2)acetyl]-l-carnitine in liver tricarboxylic acid cycle and lipogenesis: a study by 13 C NMR spectroscopy in conscious, freely moving rats. Eur J Biochem 263:287–293
Cruz F, Scott SR, Barroso I, Santisteban P, Cerdan S (1998) Ontogeny and cellular localization of the pyruvate recycling system in rat brain. J Neurochem 70:2613–2619
Kunnecke B, Cerdan S, Seelig J (1993) Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13 C NMR spectroscopy. NMR Biomed 6:264–277
Cerdan S, Kunnecke B, Seelig J (1990) Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13 C NMR. J Biol Chem 265:12916–12926
Richards EM, Rosenthal RE, Kristian T, Fiskum G (2006) Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med 40:1960–1970
Martin E, Rosenthal RE, Fiskum G (2005) Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res 79:240–247
Bogaert YE, Sheu KF, Hof PR, Brown AM, Blass JP, Rosenthal RE, Fiskum G (2000) Neuronal subclass-selective loss of pyruvate dehydrogenase immunoreactivity following canine cardiac arrest and resuscitation. Exp Neurol 161:115–126
Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G (2006) Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab 26:821–835
Scafidi S, O’Brien J, Hopkins I, Robertson C, Fiskum G, McKenna M (2009) Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J Neurochem 109(Suppl 1):189–197
Hassel B, Sonnewald U, Fonnum F (1995) Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13 C NMR spectroscopic study. J Neurochem 64:2773–2782
Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23:5928–5935
Aureli T, Di Cocco ME, Puccetti C, Ricciolini R, Scalibastri M, Miccheli A, Manetti C, Conti F (1998) Acetyl-L-carnitine modulates glucose metabolism and stimulates glycogen synthesis in rat brain. Brain Res 796:75–81
Ori C, Freo U, Pizzolato G, Dam M (2002) Effects of acetyl-l-carnitine on regional cerebral glucose metabolism in awake rats. Brain Res 951:330–335
Waniewski RA, Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 18:5225–5233
Bigford GE, Del Rossi G (2014) Supplemental substances derived from foods as adjunctive therapeutic agents for treatment of neurodegenerative diseases and disorders. Adv Nutr 5:394–403
Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield DA, Calvani M, Pennisi G, Giuffrida Stella AM (2003) Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res 28:1321–1328
Markowska AL, Ingram DK, Barnes CA, Spangler EL, Lemken VJ, Kametani H, Yee W, Olton DS (1990) Acetyl-1-carnitine. 1: effects on mortality, pathology and sensory-motor performance in aging rats. Neurobiol Aging 11:491–498
Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS (1990) Acetyl-1-carnitine. 2: effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol Aging 11:499–506
Kobayashi S, Iwamoto M, Kon K, Waki H, Ando S, Tanaka Y (2010) Acetyl-l-carnitine improves aged brain function. Geriatr Gerontol Int 10(Suppl 1):S99–S106
Al-Majed AA, Sayed-Ahmed MM, Al-Omar FA, Al-Yahya AA, Aleisa AM, Al-Shabanah OA (2006) Carnitine esters prevent oxidative stress damage and energy depletion following transient forebrain ischaemia in the rat hippocampus. Clin Exp Pharmacol Physiol 33:725–733
McKenna MC, Scafidi S, Robertson CL (2015) Metabolic alterations in developing brain after injury: knowns and unknowns. Neurochem Res 40:2527–2543
Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, Ferriero DM, Guillet R, Gunn AJ, Hagberg H, Hirtz D, Inder TE, Jacobs SE, Jenkins D, Juul S, Laptook AR, Lucey JF, Maze M, Palmer C, Papile L, Pfister RH, Robertson NJ, Rutherford M, Shankaran S, Silverstein FS, Soll RF, Thoresen M, Walsh WF (2011) Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 159(5):851–858.e1
Fatemi A, Wilson MA, Johnston MV (2009) Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol 36(4):835–858, vii
Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE (2008) A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol 199:587–595
Rice JE 3rd, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Smith AL, Rosenkrantz TS, Fitch RH (2016) Effects of sex and mild intrainsult hypothermia on neuropathology and neural reorganization following neonatal hypoxic ischemic brain injury in rats. Neural Plast 2016:2585230
Morken TS, Brekke E, Haberg A, Wideroe M, Brubakk AM, Sonnewald U (2014) Altered astrocyte-neuronal interactions after hypoxia-ischemia in the neonatal brain in female and male rats. Stroke 45:2777–2785
Chavez-Valdez R, Martin LJ, Razdan S, Gauda EB, Northington FJ (2014) Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience 260:106–119
Brekke EM, Morken TS, Wideroe M, Haberg AK, Brubakk AM, Sonnewald U (2014) The pentose phosphate pathway and pyruvate carboxylation after neonatal hypoxic-ischemic brain injury. J Cereb Blood Flow Metab 34:724–734
Osredkar D, Sall JW, Bickler PE, Ferriero DM (2010) Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis 38:259–265
de Paula S, Vitola AS, Greggio S, de Paula D, Mello PB, Lubianca JM, Xavier LL, Fiori HH, Dacosta JC (2009) Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr Res 65:631–635
Yang J, Liu J, Niu G, Liu Y, Wu EX (2008) Magnetic resonance imaging of migrating neuronal precursors in normal and hypoxic-ischemic neonatal rat brains by intraventricular MPIO labeling. Conf Proc IEEE Eng Med Biol Soc 2008:363–366
Demarest TG, Schuh RA, Waite EL, Waddell J, McKenna MC, Fiskum G (2016) Sex dependent alterations in mitochondrial electron transport chain proteins following neonatal rat cerebral hypoxic-ischemia. J Bioenerg Biomembr 48:591–598
Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G (2016) Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem 137:714–729
Faul M, Xu L, Wald MM, Coronado VG, Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. 2010. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta
Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N (2004) Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc 10:412–426
Wechsler B, Kim H, Gallagher PR, DiScala C, Stineman MG (2005) Functional status after childhood traumatic brain injury. J Trauma 58:940–949 discussion 950.
Ryan NP, Anderson V, Godfrey C, Beauchamp MH, Coleman L, Eren S, Rosema S, Taylor K, Catroppa C (2014) Predictors of very-long-term sociocognitive function after pediatric traumatic brain injury: evidence for the vulnerability of the immature “social brain”. J Neurotrauma 31:649–657
Catroppa C, Godfrey C, Rosenfeld JV, Hearps SS, Anderson VA (2012) Functional recovery ten years after pediatric traumatic brain injury: outcomes and predictors. J Neurotrauma 29:2539–2547
Babikian T, Asarnow R (2009) Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 23:283–296
Zhang X, Liu S, Newport GD, Paule MG, Callicott R, Thompson J, Liu F, Patterson TA, Berridge MS, Apana SM, Brown CC, Maisha MP, Hanig JP, Slikker W Jr, Wang C (2016) In vivo monitoring of sevoflurane-induced adverse effects in neonatal nonhuman primates using small-animal positron emission tomography. Anesthesiology 125:133–146
Zhang X, Liu S, MG P, Newport GD, Callicott R, Berridge MS, Apana SM, Jr SW, C W (2013) Protective effects of acetyl l-carnitine on inhalation anesthetic-induced neuronal damage in the nonhuman primate. J Mol Pharm Org Process Res doi:10.4172/2329-9029.1000102
Zou X, Sadovova N, Patterson TA, Divine RL, Hotchkiss CE, Ali SF, Hanig JP, Paule MG, Slikker W Jr, Wang C (2008) The effects of l-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience 151:1053–1065
Walters JL, Paule MG (2017) Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 60:2–23
De Simone R, Ramacci MT, Aloe L (1991) Effect of acetyl-l-carnitine on forebrain cholinergic neurons of developing rats. Int J Dev Neurosci 9:39–46
Piovesan P, Quatrini G, Pacifici L, Taglialatela G, Angelucci L (1995) Acetyl-l-carnitine restores choline acetyltransferase activity in the hippocampus of rats with partial unilateral fimbria-fornix transection. Int J Dev Neurosci 13:13–19
Picconi B, Barone I, Pisani A, Nicolai R, Benatti P, Bernardi G, Calvani M, Calabresi P (2006) Acetyl-l-carnitine protects striatal neurons against in vitro ischemia: the role of endogenous acetylcholine. Neuropharmacology 50:917–923
Imperato A, Ramacci MT, Angelucci L (1989) Acetyl-l-carnitine enhances acetylcholine release in the striatum and hippocampus of awake freely moving rats. Neurosci Lett 107:251–255
Mitsushima D, Sano A, Takahashi T (2013) A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun 4:2760
Gold PE (2003) Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem 80:194–210
Sarter M, Bruno JP, Givens B (2003) Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem 80:245–256
Phillis JW (2005) Acetylcholine release from the central nervous system: a 50-year retrospective. Crit Rev Neurobiol 17:161–217
Furukawa S, Yang L, Sameshima H (2014) Galantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn rats. Int J Dev Neurosci 37:52–57
Furukawa S, Yang L, Sameshima H, Ikenoue T (2014) Repetitive administration of acetylcholine receptor agonist rescues brain inflammation and brain damage after hypoxia-ischemia in newborn rat. J Perinat Med 42:379–384
Furukawa S, Sameshima H, Yang L, Harishkumar M, Ikenoue T (2014) Regional differences of microglial accumulation within 72 h of hypoxia-ischemia and the effect of acetylcholine receptor agonist on brain damage and microglial activation in newborn rats. Brain Res 1562:52–58
Juul SE, Ferriero DM (2014) Pharmacologic neuroprotective strategies in neonatal brain injury. Clin Perinatol 41:119–131
Kochanek PM, Bell MJ, Bayir H (2010) Quo vadis 2010?-carpe diem: challenges and opportunities in pediatric traumatic brain injury. Dev Neurosci 32:335–342
Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents–second edition. Pediatr Crit Care Med 13(Suppl 1):S1–S82
Kochanek PM, Jackson TC, Ferguson NM, Carlson SW, Simon DW, Brockman EC, Ji J, Bayir H, Poloyac SM, Wagner AK, Kline AE, Empey PE, Clark RS, Jackson EK, Dixon CE (2015) Emerging therapies in traumatic brain injury. Semin Neurol 35:83–100
Acknowledgements
The authors would like to gratefully acknowledge and thank Bruna Klippel Ferreira for her excellent effort in preparing Figs. 2 and 3, and Dr. Jaylyn Waddell and Dr. Susanna Scafidi for their very helpful suggestions. Research described from Dr. McKenna’s laboratory was supported in part by NIH Grants 5P01 HD016596 and P01 HD085928. Dr. Ferreira’s research is supported by the “Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro”, Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gustavo C. Ferreira and Mary C. McKenna contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ferreira, G.C., McKenna, M.C. l-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem Res 42, 1661–1675 (2017). https://doi.org/10.1007/s11064-017-2288-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2288-7