Abstract
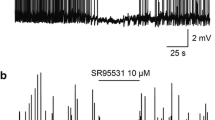
The ventrolateral preoptic nucleus is a sleep-promoting nucleus located in the basal forebrain. A commonly used intravenous anesthetic, propofol, had been reported to induce sleep spindles and augment the firing rate of neurons in ventrolateral preoptic nucleus, but the underlining mechanism is yet to be known. By using patch clamp recording on neuron in acute brain slice, present study tested if histaminergic H1 and H2 receptors play a role in the effect of propofol on the noradrenalin-inhibited neurons in ventrolateral preoptic nucleus. We found that the firing rate of noradrenalin-inhibited neurons were significantly augmented by propofol; the frequency of inhibitory postsynaptic currents of noradrenalin-inhibited neuron were evidently attenuated by propofol; such inhibition effect was suppressed by histamine; and both triprolidine (antagonist for H1 histamine receptor) and ranitidine (antagonist for H2 histamine receptor) were able to increase the inhibition rate of propofol in presence of histamine. Present study demonstrated that propofol-induced inhibition of inhibitory postsynaptic currents on noradrenalin-inhibited neurons were mediated by histaminergic H1 and H2 receptors.




Similar content being viewed by others
References
Hutt A (2013) The anesthetic propofol shifts the frequency of maximum spectral power in EEG during general anesthesia: analytical insights from a linear model. Front Comput Neurosci 7:2
Nicolaou N, Hourris S, Alexandrou P, Georgiou J (2012) EEG-based automatic classification of ‘awake’ versus ‘anesthetized’ state in general anesthesia using Granger causality. PloS one 7:e33869
Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386
Hsieh KC, Gvilia I, Kumar S, Uschakov A, McGinty D, Alam MN, Szymusiak R (2011) c-Fos expression in neurons projecting from the preoptic and lateral hypothalamic areas to the ventrolateral periaqueductal gray in relation to sleep states. Neuroscience 188:55–67
Liu YW, Zuo W, Ye JH (2013) Propofol stimulates noradrenalin-inhibited neurons in the ventrolateral preoptic nucleus by reducing GABAergic inhibition. Anesth Analg 117:358–363
Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB (2012) Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol 22:2008–2016
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Liu YW, Li J, Ye JH (2010) Histamine regulates activities of neurons in the ventrolateral preoptic nucleus. J Physiol 588:4103–4116
Zecharia AY, Yu X, Gotz T, Ye Z, Carr DR, Wulff P, Bettler B, Vyssotski AL, Brickley SG, Franks NP, Wisden W (2012) GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J Neurosci 32:13062–13075
Zhang Y, He JC, Liu XK, Zhang Y, Wang Y, Yu T (2014) Assessment of the effect of etomidate on voltage-gated sodium channels and action potentials in rat primary sensory cortex pyramidal neurons. Eur J Pharmacol 736:55–62
Buldakova SL, Tikhonov DB, Magazanik LG (2005) Analysis of the excitatory and inhibitory components of postsynaptic currents recorded in pyramidal neurons and interneurons in the rat hippocampus. Neurosci Behav Physiol 35:835–843
Babateen O, Jin Z, Bhandage A, Korol SV, Westermark B, Forsberg Nilsson K, Uhrbom L, Smits A, Birnir B (2015) Etomidate, propofol and diazepam potentiate GABA-evoked GABAA currents in a cell line derived from human glioblastoma. Eur J Pharmacol 748:101–107
Nishikawa K, Kubo K, Obata H, Yanagawa Y, Saito S (2011) The influence of manipulations to alter ambient GABA concentrations on the hypnotic and immobilizing actions produced by sevoflurane, propofol, and midazolam. Neuropharmacology 61:172–180
Yanovsky Y, Schubring S, Fleischer W, Gisselmann G, Zhu XR, Lubbert H, Hatt H, Rudolph U, Haas HL, Sergeeva OA (2012) GABAA receptors involved in sleep and anaesthesia: beta1- versus beta3-containing assemblies. Pflug Arch 463:187–199
Fagerlund MJ, Sjodin J, Krupp J, Dabrowski MA (2010) Reduced effect of propofol at human {alpha}1{beta}2(N289M){gamma}2 and {alpha}2{beta}3(N290M){gamma}2 mutant GABA(A) receptors. Br J Anaesth 104:472–481
Jin X, Zhong W, Jiang C (2013) Time-dependent modulation of GABA(A)-ergic synaptic transmission by allopregnanolone in locus coeruleus neurons of Mecp2-null mice. Am J Physiol Cell Physiol 305:C1151–C1160
Rotaru DC, Olezene C, Miyamae T, Povysheva NV, Zaitsev AV, Lewis DA, Gonzalez-Burgos G (2015) Functional properties of GABA synaptic inputs onto GABA neurons in monkey prefrontal cortex. J Neurophysiol 113:1850–1861
Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z, Uygun DS, Parker S, Vyssotski AL, Yustos R, Franks NP, Brickley SG, Wisden W (2015) Wakefulness is governed by GABA and histamine cotransmission. Neuron 87:164–178
Strecker RE, Nalwalk J, Dauphin LJ, Thakkar MM, Chen Y, Ramesh V, Hough LB, McCarley RW (2002) Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep-wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience 113:663–670
Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM (2006) Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol 96:1581–1591
Acknowledgements
This work was supported by a Grant from National Natural Science Foundation of China (81460219 and 81571026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Yang Liu and Yu Zhang have equally contributed on this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, Y., Qian, K. et al. Histaminergic H1 and H2 Receptors Mediate the Effects of Propofol on the Noradrenalin-Inhibited Neurons in Rat Ventrolateral Preoptic Nucleus. Neurochem Res 42, 1387–1393 (2017). https://doi.org/10.1007/s11064-017-2187-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2187-y