Abstract
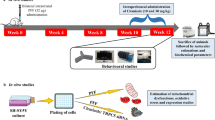
Dopamine D3 receptor (DRD3) is diminished in patients of Parkinson’s disease (PD). Brain-derived neurotrophic factor (BDNF) is responsible for regulating expression of the DRD3 in the brain. Our previous study showed that hydroxysafflor yellow A (HSYA) could increase BDNF content in the striatum of PD mice. This experiment aimed to evaluate whether HSYA can improve the motor dysfunction induced by rotenone through regulating the BDNF/TrkB/DRD3 signaling pathway in mice. Male C57/BL6 mice were intraperitoneally treated with HSYA. Thirty minutes later, they were intragastrically administered with rotenone at a dose of 30 mg/kg. Pole, rotarod and open field tests were investigated at 28 d. Then, tyrosine hydroxylase (TH) in substantia nigra was observed by immunohistochemistry. Dopamine content was detected by high-performance liquid chromatography. The expressions of BDNF, phospho-tropomyosin-related kinase B (p-TrkB), tropomyosin-related kinase B (TrkB), phospho-phosphoinositide 3-kinase (p-PI3K), phosphoinositide 3-kinase (PI3K), phospho-protein kinase B (p-AKT), protein kinase B (AKT), and DRD3 were assayed by western blotting. Behavioral tests showed that rotenone-challenged mice displayed motor dysfunction. However, treatment with HSYA improved motor dysfunction induced by rotenone. HSYA treatment increased not only the number of TH-containing dopaminergic neurons in substantia nigra, but also the dopamine content in the striatum in PD mice. Moreover, the expressions of BDNF, p-TrkB/TrkB, DRD3, p-PI3K/PI3K, p-AKT/AKT were significantly increased in rotenone plus HSYA group. Our results indicated that HSYA improved motor dysfunction in rotenone-induced PD model and the pharmacological action of HSYA was related to regulating BDNF/TrkB/DRD3 signaling pathway, at least, in part.




Similar content being viewed by others
References
Han B, Hu J, Shen J, Gao Y, Lu Y, Wang T (2013) Neuroprotective effect of hydroxysafflor yellow A on 6-hydroxydopamine-induced Parkinson’s disease in rats. Eur J Pharmacol 714(1–3):83–88. doi:10.1016/j.ejphar.2013.06.011
Heng Y, Zhang QS, Mu Z, Hu JF, Yuan YH, Chen NH (2016) Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol Lett 243:7–21. doi:10.1016/j.toxlet.2015.12.005
Noelker C, Morel L, Osterloh A, Alvarez-Fischer D, Lescot T, Breloer M, Gold M, Oertel WH, Henze C, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A (2014) Heat shock protein 60: an endogenous inducer of dopaminergic cell death in Parkinson disease. J Neuroinflammation 11:86. doi:10.1186/1742-2094-11-86
Zhang S, Shao SY, Song XY, Xia CY, Yang YN, Zhang PC, Chen NH (2016) Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology 52:72–83. doi:10.1016/j.neuro.2015.09.009
Wang T, Duan SJ, Wang SY, Lu Y, Zhu Q, Wang LJ, Han B (2015) Coadministration of hydroxysafflor yellow A with levodopa attenuates the dyskinesia. Physiol Behav 147:193–197. doi:10.1016/j.physbeh.2015.04.038
Das B, Modi G, Dutta A (2015) Dopamine D3 agonists in the treatment of Parkinson’s disease. Curr Top Med Chem 15(10):908–926
Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, Mulsant B, Pollock B, Graff-Guerrero A (2013) The potential role of dopamine D(3) receptor neurotransmission in cognition. European Neuropsychopharmacol 23(8):799–813. doi:10.1016/j.euroneuro.2013.05.006
Kassel S, Schwed JS, Stark H (2015) Dopamine D3 receptor agonists as pharmacological tools. European Neuropsychopharmacol 25(9):1480–1499. doi:10.1016/j.euroneuro.2014.11.005
Carnicella S, Drui G, Boulet S, Carcenac C, Favier M, Duran T, Savasta M (2014) Implication of dopamine D3 receptor activation in the reversion of Parkinson’s disease-related motivational deficits. Transl Psychiatr 4:e401. doi:10.1038/tp.2014.43
Levesque D, Martres MP, Diaz J, Griffon N, Lammers CH, Sokoloff P, Schwartz JC (1995) A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci USA 92(5):1719–1723
Simms SL, Huettner DP, Kortagere S (2016) In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson’s disease. Neuropharmacology 100:106–115. doi:10.1016/j.neuropharm.2015.04.004
Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, Bannon MJ, Martinez-Fong D, Aceves-Ruiz J (2015) The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PloS ONE 10(2):e0117391. doi:10.1371/journal.pone.0117391
Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166(1):127–135. doi:10.1006/exnr.2000.7483
Porritt M, Stanic D, Finkelstein D, Batchelor P, Lockhart S, Hughes A, Kalnins R, Howells D (2005) Dopaminergic innervation of the human striatum in Parkinson’s disease. Mov Disord 20(7):810–818. doi:10.1002/mds.20399
White FJ (2001) Neurobiology. Dopamine receptors get a boost. Nature 411(6833):35–38. doi:10.1038/35075193
Yoshii A, Constantine-Paton M (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Develop Neurobiol 70(5):304–322. doi:10.1002/dneu.20765
Yang Y, Qiu WQ, Hao YL, Lv ZY, Jiao SJ, Teng JF (2015) The efficacy of traditional Chinese medical exercise for Parkinson’s disease: a systematic review and meta-analysis. PLoS ONE 10(4):e0122469. doi:10.1371/journal.pone.0122469
Sun L, Yang L, Xu YW, Liang H, Han J, Zhao RJ, Cheng Y (2012) Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res 1473:227–235. doi:10.1016/j.brainres.2012.07.047
Han B, Zhao H (2010) Effects of hydroxysafflor yellow A in the attenuation of MPTP neurotoxicity in mice. Neurochem Res 35(1):107–113. doi:10.1007/s11064-009-0035-4
Segura-Aguilar J, Kostrzewa RM (2015) Neurotoxin mechanisms and processes relevant to Parkinson’s disease: an update. Neurotox Res 27(3):328–354. doi:10.1007/s12640-015-9519-y
Lin Z, Dodd CA, Filipov NM (2013) Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol Teratol 39:26–35. doi:10.1016/j.ntt.2013.06.002
Beaulieu JM, Espinoza S, Gainetdinov RR (2015) Dopamine receptors—IUPHAR Review 13. Br J Pharmacol 172(1):1–23. doi:10.1111/bph.12906
Ren Y, Liu W, Jiang H, Jiang Q, Feng J (2005) Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem 280(40):34105–34112. doi:10.1074/jbc.M503483200
Inden M, Kitamura Y, Tamaki A, Yanagida T, Shibaike T, Yamamoto A, Takata K, Yasui H, Taira T, Ariga H, Taniguchi T (2009) Neuroprotective effect of the antiparkinsonian drug pramipexole against nigrostriatal dopaminergic degeneration in rotenone-treated mice. Neurochem Int 55(8):760–767. doi:10.1016/j.neuint.2009.07.009
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411(6833):86–89. doi:10.1038/35075076
Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12(10):1329–1343. doi:10.1038/sj.cdd.4401662
Inden M, Kitamura Y, Abe M, Tamaki A, Takata K, Taniguchi T (2011) Parkinsonian rotenone mouse model: reevaluation of long-term administration of rotenone in C57BL/6 mice. Biological pharmaceutical bulletin 34(1):92–96
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW, Merchant KM, Bezard E, Petsko GA, Meissner WG (2015) Targeting alpha-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. The Lancet Neurology 14(8):855–866. doi:10.1016/S1474-4422(15)00006-X
Shimizu S, Tatara A, Sato M, Sugiuchi T, Miyoshi S, atsu S (2014) Role of cerebellar dopamine D (3) receptors in modulating exploratory locomotion and cataleptogenicity in rats. Prog Neuropsychopharmacol Biol Psychiatr 50:157–162. doi:10.1016/j.pnpbp.2013.12.013
Acknowledgements
This work was supported by the Joint Fund Project of the Education Department of Shandong Province (NO. ZR2014JL052) and Taishan Scholars Program of Shandong Province (NO. TSHW201502046). The authors are grateful to Prof. Lon Clark for his assistance in English language revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to declare.
Additional information
Tian Wang and Lijie Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, T., Wang, L., Li, C. et al. Hydroxysafflor Yellow A Improves Motor Dysfunction in the Rotenone-Induced Mice Model of Parkinson’s Disease. Neurochem Res 42, 1325–1332 (2017). https://doi.org/10.1007/s11064-017-2176-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2176-1