Abstract
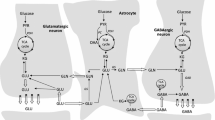
There is compelling evidence that initiation and maintenance of epileptic seizures in temporal lobe epilepsy (TLE) is facilitated by excessive accumulation in the extracellular (perisynaptic) space of the excitatory neurotransmitter glutamate (Glu). This review discusses the mechanisms underlying this phenomenon. Glu released from neurons is taken up by astrocytes and activated there by glutamine synthetase (GS) to form glutamine (Gln) which upon entry to neurons is degraded back to Glu by phosphate-activated glutaminase (PAG): this chain of reactions has been defined as the glutamine/glutamate/cycle (GGC). In the initial phase of epileptogenesis, increased Glu supply is a consequence of activation of its turnover in GGC by Glu released by a primary chemical or physical stimulus. In chronic TLE, profound astrogliosis and demise of neurons which culminate in hippocampal sclerosis, are associated with changes in GGC which act in concert towards increasing the extracellular Glu concentration. Deficiency of GS and of the astrocytic Glu transporter, GLT-1, impede Glu inactivation, whereas Glu release from neurons appears facilitated by activation of PAG and increased activity of the neuronal Glu transporter EAAC1. Conclusions derived from measurements of activities/expression patterns of the GGC enzymes and transporter moieties find support in metabolic studies employing 13C labeled Glu precursors. Glu reuptake by astrocytes is additionally impeded by unfavorable ion gradients resulting from ion and water dyshomeostasis, and extracellular Glu concentration is further increased by reduction of extracellular space due to edema and altered cytoarchitecture of the hippocampus. Missing links in the scenario are discussed in concluding comments.

Similar content being viewed by others
References
Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M (1998) Is the underlying cause of epilepsy a major prognostic factor for recurrence?. Neurology 51:1256–1262
Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R, Kroner B, Labiner D, Liow K, Logroscino G, Medina MT, Newton CR, Parko K, Paschal A, Preux PM, Sander JW, Selassie A, Theodore W, Tomson T, Wiebe S (2011) Standards for epidemiologic studies and surveillance of epilepsy. ILAE Commission on Epidemiology. Epilepsia 52(Suppl 7):2–26. doi:10.1111/j.1528-1167.2011.03121.x
French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD (1993) Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol 34:774–780
Seinfeld S, Goodkin HP, Shinnar S (2016) Status Epilepticus. Cold Spring Harb Perspect Med6:a022830. doi:10.1101/cshperspect.a022830
Sloviter RS (2008) Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia 49(Suppl 9):85–92. doi:10.1111/j.1528-1167.2008.01931.x
Puttachary S, Sharma S, Tse K, Beamer E, Sexton A, Crutison J, Thippeswamy T (2015) Immediate epileptogenesis after kainate-induced status epilepticus in C57BL/6J mice: evidence from long term continuous video-eeg telemetry. PLoS One 10(10):e0131705. doi:10.1371/journal.pone.0131705
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L (1991) Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia 32:778–782
Seifert G, Steinhäuser C (2013) Neuron-astrocyte signaling and epilepsy. Exp Neurol 244:4–10. doi:10.1016/j.expneurol.2011.08.024
Thom M (2014) Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 40:520–543. doi:10.1111/nan.12150
Barker-Haliski M, White HS (2015) Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 5:a022863. doi:10.1101/cshperspect.a022863
Robel S, Sontheimer H (2016) Glia as drivers of abnormal neuronal activity. Nat Neurosci 19:28–33. doi:10.1038/nn.4184
Crunelli V, Carmignoto G (2013) New vistas on astroglia in convulsive and non-convulsive epilepsy highlight novel astrocytic targets for treatment. J Physiol 591:775–785
Olney JW, Collins RC, Sloviter RS (1986) Excitototoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. The Lancet 341:1607–1610
Thomas PM, Phillips JP, O’Connor WT (2004) Hippocampal microdialysis during spontaneous intraoperative epileptiform activity. Acta Neurochir (Wien) 146:143–151
Çavuş I, Romanyshyn JC, Kennard JT, Farooque P, Williamson A, Eid T, Spencer SS, Duckrow R, Dziura J, Spencer DD (2016) Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: microdialysis study of 79 patients at the Yale epilepsy surgery program. Ann Neurol 80:35–45. doi:10.1002/ana.24673
Millan MH, Chapman AG, Meldrum BS (1993) Extracellular amino acid levels in hippocampus during pilocarpine-induced seizures. Epilepsy Res 14:139–148
Kanamori K, Ross BD (2013) Electrographic seizures are significantly reduced by in vivo inhibition of neuronal uptake of extracellular glutamine in rat hippocampus. Epilepsy Res 107:20–36. doi:10.1016/j.eplepsyres.2013.08.007
Khan GM, Smolders I, Lindekens H, Manil J, Ebinger G, Michotte Y (1999) Effects of diazepam on extracellular brain neurotransmitters in pilocarpine-induced seizures in rats. Eur J Pharmacol 373:153–161
Kanamori K, Ross BD (2011) Chronic electrographic seizure reduces glutamine and elevates glutamate in the extracellular fluid of rat brain. Brain Res 1371:180–191. doi:10.1016/j.brainres.2010.11.064
Dulla C, Tani H, Okumoto S, Frommer WB, Reimer RJ, Huguenard JR (2008) Imaging of glutamate in brain slices using FRET sensors. J Neurosci Methods 168:306–319
Tani H, Dulla CG, Huguenard JR, Reimer RJ (2010) Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J Neurosci 30:1288–1300. doi:10.1523/JNEUROSCI.0106-09.2010
van der Hel WS, Verlinde SA, Meijer DH, de Wit M, Rensen MG, van Gassen KL, van Rijen PC, van Veelen CW, de Graan PN (2009) Hippocampal distribution of vesicular glutamate transporter 1 in patients with temporal lobe epilepsy. Epilepsia 50:1717–1728. doi:10.1111/j.1528-1167.2009.02054.x
Wasterlain CG, Naylor DE, Liu H, Niquet J, Baldwin R (2013) Trafficking of NMDA receptors during status epilepticus: therapeutic implications. Epilepsia 54(Suppl 6):78–80
Frasca A, Aalbers M, Frigerio F, Fiordaliso F, Salio M, Gobbi M, Cagnotto A, Gardoni F, Battaglia GS, Hoogland G, Di Luca M, Vezzani A (2011) Misplaced NMDA receptors in epileptogenesis contribute to excitotoxicity. Neurobiol Dis 43:507–515. doi:10.1016/j.nbd.2011.04.024
Lopes MW, Soares FMS, de Mello N, Nunes JC, Cajado AG, de Brito D, de Cordova FM, Silva da Cunha MRS, Walz W, Leal RB (2013) Time-dependent modulation of AMPA receptor phosphorylation and mRNA expression of NMDA receptors and glial glutamate transporters in the rat hippocampus and cerebral cortex in a pilocarpine model of epilepsy. Exp Brain Res 226:153–163. doi:10.1007/s00221-013-3421-8
Das A, Wallace GC 4th, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK, Banik NL (2012) Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 220:237–246. doi:10.1016/j.neuroscience.2012.06.002
McGinnity CJ, Koepp MJ, Hammers A, Riaño Barros DA, Pressler RM, Luthra S, Jones PA, Trigg W, Micallef C, Symms MR, Brooks DJ, Duncan JS (2015) NMDA receptor binding in focal epilepsies. J Neurol Neurosurg Psychiatry 86:1150–1157. doi:10.1136/jnnp-2014-309897
Schousboe A, Bak LK, Waagepetersen HS (2013) Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 4:102. doi:10.3389/fendo.2013.00102
Crunelli V, Cope DW, Terry JR (2011) Transition to absence seizures and the role of GABA(A) receptors. Epilepsy Res 97:283–289. doi:10.1016/j.eplepsyres.2011.07.011
van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN (2005) Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology 64:326–333
Eid T, Thomas MJ, Spencer DD, Rundén-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC (2004) Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 363:28–37
van der Hel WS, Hessel EV, Bos IW, Mulder SD, Verlinde SA, van Eijsden P, de Graan PN (2014) Persistent reduction of hippocampal glutamine synthetase expression after status epilepticus in immature rats. Eur J Neurosci 40:3711–3719. doi:10.1111/ejn.12756
Swamy M, Yusof WR, Sirajudeen KN, Mustapha Z, Govindasamy C (2011) Decreased glutamine synthetase, increased citrulline-nitric oxide cycle activities, and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem 67:105–113. doi:10.1007/s13105-010-0054-2
Eid T, Ghosh A, Wang Y, Beckström H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC (2008) Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain 131:2061–2070. doi:10.1093/brain/awn133
Rosati A, Marconi S, Pollo B, Tomassini A, Lovato L, Maderna E, Maier K, Schwartz A, Rizzuto N, Padovani A, Bonetti B (2009) Epilepsy in glioblastoma multiforme: correlation with glutamine synthetase levels. J Neurooncol 93:319–324. doi:10.1007/s11060-008-9794-z
Bidmon HJ, Görg B, Palomero-Gallagher N, Schleicher A, Häussinger D, Speckmann EJ, Zilles K (2008) Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia 49:1733–1748. doi:10.1111/j.1528-1167.2008.01642.x
Chen SD, Chang AY, Chuang YC (2010) The potential role of mitochondrial dysfunction in seizure-associated cell death in the hippocampus and epileptogenesis. J Bioenerg Biomembr 42:461–465. doi:10.1007/s10863-010-9321-8
Zsurka G, Kunz WS (2015) Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol 14:956–966. doi:10.1016/S1474-4422(15)00148-9
Papageorgiou IE, Gabriel S, Fetani AF, Kann O, Heinemann U (2011) Redistribution of astrocytic glutamine synthetase in the hippocampus of chronic epileptic rats. Glia 59:1706–1718. doi:10.1002/glia.21217
Hammer J, Alvestad S, Osen KK, Skare Ø, Sonnewald U, Ottersen OP (2008) Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia 56:856–868. doi:10.1002/glia.20659
Hubbard JA, Szu JI, Yonan JM, Binder DK (2016) Regulation of astrocyte glutamate transporter-1 (GLT1) and aquaporin-4 (AQP4) expression in a model of epilepsy. Exp Neurol 283:85–96. doi:10.1016/j.expneurol.2016.05.003
Sakurai M, Kurokawa H, Shimada A, Nakamura K, Miyata H, Morita T (2015) Excitatory amino acid transporter 2 downregulation correlates with thalamic neuronal death following kainic acid-induced status epilepticus in rat. Neuropathology 35:1–9. doi:10.1111/neup.12141
Kong Q, Takahashi K, Schulte D, Stouffer N, Lin Y, Lin CL (2012) Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiol Dis 47:145–154. doi:10.1016/j.nbd.2012.03.032
Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS (2008) Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med (Maywood) 233:1389–1394. doi:10.3181/0803-RM-83
Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN (2002) Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 125:32–43
Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, Troost D, de Graan PN (2004) Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res 59:75–82
Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC (1999) Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience 88:1083–1091
Bjørnsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC (2007) Changes in glial glutamate transporters in human epileptogenic hippocampus: inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis 25:319–330
Sun HL, Zhang SH, Zhong K, Xu ZH, Feng B, Yu J, Fang Q, Wang S, Wu DC, Zhang JM, Chen Z (2013) A transient upregulation of glutamine synthetase in the dentate gyrus is involved in epileptogenesis induced by amygdala kindling in the rat. PLoS One 8:e66885. doi:10.1371/journal.pone.0066885
Takahashi DK, Vargas JR, Wilcox KS (2010) Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis 40:573–585
Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U (1997) Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab 17:1230–1238
Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, Foutz AS, Parrot, S, Miller GM, Jorisch R (2006) Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci 26:4660–4671
Albrecht J, Sidoryk-Węgrzynowicz M, Zielińska M, Aschner M (2011) Roles of glutamine in neurotransmission. Neuron Glia Biol 6:263–276. doi:10.1017/S1740925X11000093
Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M (2002) Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol 88:2302–2310
Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ (2014) A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81:888–900. doi:10.1016/j.neuron.2013.12.026
Tani H, Bandrowski AE, Parada I, Wynn M, Huguenard JR, Prince DA, Reimer RJ (2007) Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy. Neurobiol Dis 25:230–238
Kanamori K (2015) Disinhibition reduces extracellular glutamine and elevates extracellular glutamate in rat hippocampus in vivo. Epilepsy Res 114:32–46. doi:10.1016/j.eplepsyres.2015.03.009
Billups D, Marx MC, Mela I, Billups B (2013) Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J Neurosci 33:17429–17434. doi:10.1523/JNEUROSCI.1466-13.2013
Eid T, Hammer J, Rundén-Pran E, Roberg B, Thomas MJ, Osen K, Davanger S, Laake P, Torgner IA, Lee TS, Kim JH, Spencer DD, Ottersen OP, de Lanerolle NC (2007) Increased expression of phosphate-activated glutaminase in hippocampal neurons in human mesial temporal lobe epilepsy. Acta Neuropathol 113:137–152
Eid T, Lee TS, Wang Y, Perez E, Drummond J, Lauritzen F, Bergersen LH, Meador-Woodruff JH, Spencer DD, de Lanerolle NC, McCullumsmith RE (2013) Gene expression of glutamate metabolizing enzymes in the hippocampal formation in human temporal lobe epilepsy. Epilepsia 54:228–238. doi:10.1111/epi.12008
Doi T, Ueda Y, Takaki M, Willmore LJ (2011) Differential molecular regulation of glutamate in kindling resistant rats. Brain Res 1375:1–6. doi:10.1016/j.brainres.2010.11.085
Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S (2006) The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem 98:1007–1018
Gebhardt C, Körner R, Heinemann U (2002) Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. J Cereb Blood Flow Metab 22:569–575
Ross JR, Porter BE, Buckley PT, Eberwine JH, Robinson MB (2011) mRNA for the EAAC1 subtype of glutamate transporter is present in neuronal dendrites in vitro and dramatically increases in vivo after a seizure. Neurochem Int 58:366–375. doi:10.1016/j.neuint.2010.12.012
Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR (2002) Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia 43:211–218
Lane MC, Jackson JG, Krizman EN, Rothstein JD, Porter BE, Robinson MB (2014) Genetic deletion of the neuronal glutamate transporter, EAAC1, results in decreased neuronal death after pilocarpine-induced status epilepticus. Neurochem Int 73:152–158. doi:10.1016/j.neuint.2013.11.013
Hadera MG, Eloqayli H, Jaradat S, Nehlig A, Sonnewald U (2015) Astrocyte-neuronal interactions in epileptogenesis. J Neurosci Res 93:1157–1164. doi:10.1002/jnr.23584
Melø TM, Nehlig A, Sonnewald U (2005) Metabolism is normal in astrocytes in chronically epileptic rats: a (13)C NMR study of neuronal-glial interactions in a model of temporal lobe epilepsy. J Cereb Blood Flow Metab 25:1254–1264
Alvestad S, Hammer J, Qu H, Håberg A, Ottersen OP, Sonnewald U (2011) Reduced astrocytic contribution to the turnover of glutamate, glutamine, and GABA characterizes the latent phase in the kainate model of temporal lobe epilepsy. J Cereb Blood Flow Metab 31:1675–1686. doi:10.1038/jcbfm.2011.36
Walls AB, Eyjolfsson EM, Schousboe A, Sonnewald U, Waagepetersen HS (2014) A subconvulsive dose of kainate selectively compromises astrocytic metabolism in the mouse brain in vivo. J Cereb Blood Flow Metab 34:1340–1346. doi:10.1038/jcbfm.2014
Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM (2005) Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57:226–235
Smeland OB, Hadera MG, McDonald TS, Sonnewald U, Borges K (2013) Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J Cereb Blood Flow Metab 33:1090–1097. doi:10.1038/jcbfm.2013.54
Petroff OA, Cavus I, Kim JH, Spencer DD (2004) Interictal extracellular glutamate concentrations are increased in hippocampal sclerosis. Ann Neurol 56:43
Vielhaber S, Niessen HG, Debska-Vielhaber G, Kudin AP, Wellmer J, Kaufmann J, Schönfeld MA, Fendrich R, Willker W, Leibfritz D, Schramm J, Elger CE, Heinze HJ, Kunz WS (2008) Subfield-specific loss of hippocampal N-acetyl aspartate in temporal lobe epilepsy. Epilepsia 49:40–50
Qu H, Eloqayli H, Müller B, Aasly J, Sonnewald U (2003) Glial-neuronal interactions following kainate injection in rats. Neurochem Int 42:101–106
Malarkey EB, Parpura V (2008) Mechanisms of glutamate release from astrocytes. Neurochem Int 52:142–154
Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ (2007) Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 55:274–281
Djukic B, Casper KB, Philpot BD, Chin L-S, McCarthy KD (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake and enhanced short-term synaptic potentiation. J Neurosci 27:11354–11365
Dvorzhak A, Vagner T, Kirmse K, Grantyn R (2016) Functional indicators of glutamate transport in single striatal astrocytes and the influence of Kir4.1 in normal and Huntington mice. J Neurosci 36:4959–4975. doi:10.1523/JNEUROSCI.0316-16.2016
Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML (2016) The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132:1–21. doi:10.1007/s00401-016-1553-1551
Hinterkeuser S, Schröder W, Hager G, Seifert G, Blümcke I, Elger CE, Schramm J, Steinhäuser C (2000) Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci 12:2087–2096
Inyushin M, Kucheryavykh LY, Kucheryavykh YV, Nichols CG, Buono RJ, Ferraro TN, Skatchkov SN, Eaton MJ (2010) Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia 51:1707–1713. doi:10.1111/j.1528-1167.2010.02592.x
Puttachary S, Sharma S, Verma S, Yang Y, Putra M, Thippeswamy A, Luo D, Thippeswamy T (2016) 1400W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol Dis 93:184–200. doi:10.1016/j.nbd.2016.05.013
Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, Sutor B, Sontheimer H (2015) Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345. doi:10.1523/JNEUROSCI.1574-14.2015
Kang N, Xu J, Xu Q, Nedergaard M, Kang J (2005) Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol 94:4121–4130
Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M (2005) An astrocytic basis of epilepsy. Nat Med 11:973–981
Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG (2007) Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci 27:10674–10684
Manning TJ Jr, Sontheimer H (1997) Spontaneous intracellular calcium oscillations in cortical astrocytes from a patient with intractable childhood epilepsy (Rasmussen’s encephalitis). Glia 21:332–337
Lee TS, Mane S, Eid T, Zhao H, Lin A, Guan Z, Kim JH, Schweitzer J, King-Stevens D, Weber P (2007) Gene expression in temporal lobe epilepsy is consistent with increased release of glutamate by astrocytes. Mol Med 13:1–13
Perez EL, Lauritzen F, Wang Y, Lee TS, Kang D, Zaveri HP, Chaudhry FA, Ottersen OP, Bergersen LH, Eid T (2012) Evidence for astrocytes as a potential source of the glutamate excess in temporal lobe epilepsy. Neurobiol Dis 47:331–337 doi:10.1016/j.nbd.2012.05.010
Morales I, Rodriguez M (2012) Self-induced accumulation of glutamate in striatal astrocytes and basal ganglia excitotoxicity. Glia 60:1481–1494. doi:10.1002/glia.22368
Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J (2007) Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem 282:24185–24197
Ormel L, Stensrud MJ, Chaudhry FA, Gundersen V (2012) A distinct set of synaptic-like microvesicles in astroglial cells contain VGLUT3. Glia 60:1289–1300. doi:10.1002/glia.22348
Van Liefferinge J, Jensen CJ, Albertini G, Bentea E, Demuyser T, Merckx E, Aronica E, Smolders I, Massie A (2015) Altered vesicular glutamate transporter expression in human temporal lobe epilepsy with hippocampal sclerosis. Neurosci Lett. 590:184–188. doi:10.1016/j.neulet.2015.01.080
Heinemann U (1986) Excitatory amino acids and epilepsy-induced changes in extracellular space size. Adv Exp Med Biol 203:449–460
Hattori H, Matsuoka O, Ishida H, Hisatsune S, Yamano T (2003) Magnetic resonance imaging in occipital lobe epilepsy with frequent seizures. Pediatr Neurol 28:216–218
Lee TS, Eid T, Mane S, Kim JH, Spencer DD, Ottersen OP, de Lanerolle NC (2004) Aquaporin-4 is increased in the sclerotic hippocampus in human temporal lobe epilepsy. Acta Neuropathol 108:493–502
Eid T, Lee TS, Thomas MJ, Amiry-Moghaddam M, Bjornsen LP, Spencer DD, Agre P, Ottersen OP, de Lanerolle NC (2005) Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci USA 102:1193–1198
Nagelhus EA, Mathiisen TM, Ottersen OP (2004) Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with Kir 4.1. Neuroscience 129:905–913
Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, Spencer DD (2008) Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia 49:1358–1366. doi:10.1111/j.1528-1167.2008.01603.x
Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, Edwards RH (1999) Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 99:769–780
Hamdani el H, Gudbrandsen M, Bjørkmo M, Chaudhry FA (2012) The system N transporter SN2 doubles as a transmitter precursor furnisher and a potential regulator of NMDA receptors. Glia 60:1671–1683. doi:10.1002/glia.22386 (Epub 2012 Jul 20)
Zielińska M, Dąbrowska K, Hadera MG, Sonnewald U, Albrecht J (2016) System N transporters are critical for glutamine release and modulate metabolic fluxes of glucose and acetate in cultured cortical astrocytes: changes induced by ammonia. J Neurochem 136:329–338. doi:10.1111/jnc.13376
Palaiologos G, Hertz L, Schousboe A (1988) Evidence that aspartate aminotransferase activity and ketodicarboxylate carrier function are essential for biosynthesis of transmitter glutamate. J Neurochem 51:317–320
Kam K, Nicoll R (2007) Excitatory synaptic transmission persists independently of the glutamate–glutamine cycle. J Neurosci 27:9192–9200
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Professor Ursula Sonnewald.
Rights and permissions
About this article
Cite this article
Albrecht, J., Zielińska, M. Mechanisms of Excessive Extracellular Glutamate Accumulation in Temporal Lobe Epilepsy. Neurochem Res 42, 1724–1734 (2017). https://doi.org/10.1007/s11064-016-2105-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2105-8