Abstract
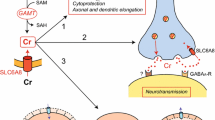
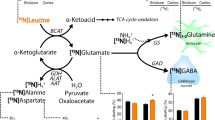
The mitochondrial aspartate/glutamate transporter Aralar/AGC1/Slc25a12 is critically involved in brain aspartate synthesis, and AGC1 deficiency results in a drastic fall of brain aspartate levels in humans and mice. It has recently been described that the uncoupling protein UCP2 transports four carbon metabolites including aspartate. Since UCP2 is expressed in several brain cell types and AGC1 is mainly neuronal, we set to test whether UCP2 could be a mitochondrial aspartate carrier in the brain glial compartment. The study of the cerebral metabolism of (1–13C)-glucose in vivo in wild type and UCP2-knockout mice showed no differences in C3 or C2 labeling of aspartate, suggesting that UCP2 does not function as a mitochondrial aspartate carrier in brain. However, surprisingly, a clear decrease (of about 30–35 %) in the fractional enrichment of glutamate, glutamine and GABA was observed in the brains of UCP2-KO mice which was not associated with differences in either glucose or lactate enrichments. The results suggest that the dilution in the labeling of glutamate and its downstream metabolites could originate from the uptake of an unlabeled substrate that could not leave the matrix via UCP2 becoming trapped in the matrix. Understanding the nature of the unlabeled substrate and its precursor(s) as alternative substrates to glucose is of interest in the context of neurological diseases associated with UCP2.



Similar content being viewed by others
References
del Arco A, Satrustegui J (1998) Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem 273(36):23327–23334
Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F (2001) Citrin and Aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. Embo J 20(18):5060–5069
Ramos M, Pardo B, Llorente-Folch I, Saheki T, Del Arco A, Satrustegui J (2011) Deficiency of the mitochondrial transporter of aspartate/glutamate Aralar/AGC1 causes hypomyelination and neuronal defects unrelated to myelin deficits in mouse brain. J Neurosci Res 89(12):2008–2017. doi:10.1002/jnr.22639
Pardo B, Rodrigues TB, Contreras L, Garzon M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrustegui J (2011) Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab 31(1):90–101. doi:10.1038/jcbfm.2010.146
Satrustegui J, Pardo B, Del Arco A (2007) Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev 87(1):29–67
Satrustegui J, Contreras L, Ramos M, Marmol P, Del Arco A, Saheki T, Pardo B (2007) Role of Aralar, the mitochondrial transporter of aspartate–glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res 85(15):3359–3366
Jalil MA, Begum L, Contreras L, Pardo B, Iijima M, Li MX, Ramos M, Marmol P, Horiuchi M, Shimotsu K, Nakagawa S, Okubo A, Sameshima M, Isashiki Y, Del Arco A, Kobayashi K, Satrustegui J, Saheki T (2005) Reduced N-acetylaspartate levels in mice lacking Aralar, a brain- and muscle-type mitochondrial aspartate–glutamate carrier. J Biol Chem 280(35):31333–31339
Urenjak J, Williams SR, Gadian DG, Noble M (1993) Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 13(3):981–989
Wibom R, Lasorsa FM, Tohonen V, Barbaro M, Sterky FH, Kucinski T, Naess K, Jonsson M, Pierri CL, Palmieri F, Wedell A (2009) AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med 361(5):489–495. doi:10.1056/NEJMoa0900591
Ricquier D, Bouillaud F (2000) The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J 345(Pt 2):161–179
Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F, Fiermonte G (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 111(3):960–965. doi:10.1073/pnas.1317400111
Ledesma A, de Lacoba MG, Rial E (2002) The mitochondrial uncoupling proteins. Genome Biol 3(12):REVIEWS3015
Krauss S, Zhang CY, Lowell BB (2005) The mitochondrial uncoupling-protein homologues. Nature Rev Mol Cell Biol 6(3):248–261. doi:10.1038/nrm1572
Nedergaard J, Cannon B (2003) The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol 88(1):65–84
Bouillaud F (2009) UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta 1787(5):377–383. doi:10.1016/j.bbabio.2009.01.003
Richard D, Clavel S, Huang Q, Sanchis D, Ricquier D (2001) Uncoupling protein 2 in the brain: distribution and function. Biochemical Society transactions 29(Pt 6):812–817
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1):264–278. doi:10.1523/JNEUROSCI.4178-07.2008
Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N (2008) Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135(4):749–762. doi:10.1016/j.cell.2008.10.029
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89(1):37–53. doi:10.1016/j.neuron.2015.11.013
Lu M, Zhao FF, Tang JJ, Su CJ, Fan Y, Ding JH, Bian JS, Hu G (2012) The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal 17(6):849–859. doi:10.1089/ars.2011.4507
Toda C, Kim JD, Impellizzeri D, Cuzzocrea S, Liu ZW, Diano S (2016) UCP2 regulates mitochondrial fission and ventromedial nucleus control of glucose responsiveness. Cell 164(5):872–883. doi:10.1016/j.cell.2016.02.010
Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB (2001) Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105(6):745–755
Cerdan S, Kunnecke B, Seelig J (1990) Cerebral metabolism of [1,2–13C2]acetate as detected by in vivo and in vitro 13 C NMR. J Biol Chem 265(22):12916–12926
Shank RP, Bennett GS, Freytag SO, Campbell GL (1985) Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 329(1–2):364–367
Shank RP, Leo GC, Zielke HR (1993) Cerebral metabolic compartmentation as revealed by nuclear magnetic resonance analysis of d-[1-13C] glucose metabolism. J Neurochem 61(1):315–323
Contreras L, Urbieta A, Kobayashi K, Saheki T, Satrustegui J (2010) Low levels of citrin (SLC25A13) expression in adult mouse brain restricted to neuronal clusters. J Neurosci Res 88(5):1009–1016. doi:10.1002/jnr.22283
Du J, Rountree A, Cleghorn WM, Contreras L, Lindsay KJ, Sadilek M, Gu H, Djukovic D, Raftery D, Satrustegui J, Kanow M, Chan L, Tsang SH, Sweet IR, Hurley JB (2016) Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J Biol Chem 291(9):4698–4710. doi:10.1074/jbc.M115.698985
Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrustegui J, Hurley JB (2014) Pyruvate kinase and aspartate–glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc Natl Acad Sci USA 111(43):15579–15584. doi:10.1073/pnas.1412441111
Contreras L (2015) Role of AGC1/Aralar in the metabolic synergies between neuron and glia. Neurochem Int. doi:10.1016/j.neuint.2015.04.001
Pardo B, Contreras L, Satrustegui J (2013) De novo synthesis of glial glutamate and glutamine in young mice requires aspartate provided by the neuronal mitochondrial aspartate–glutamate carrier Aralar/AGC1. Front Endocrinol (Lausanne) 4:149. doi:10.3389/fendo.2013.00149
Sonnewald U (2014) Glutamate synthesis has to be matched by its degradation-where do all the carbons go? J Neurochem. doi:10.1111/jnc.12812
McKenna MC, Hopkins IB, Lindauer SL, Bamford P (2006) Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem Int 48(6–7):629–636. doi:10.1016/j.neuint.2005.11.018
Karaca M, Frigerio F, Migrenne S, Martin-Levilain J, Skytt DM, Pajecka K, Martin-del-Rio R, Gruetter R, Tamarit-Rodriguez J, Waagepetersen HS, Magnan C, Maechler P (2015) GDH-dependent glutamate oxidation in the brain dictates peripheral energy substrate distribution. Cell Rep 13(2):365–375. doi:10.1016/j.celrep.2015.09.003
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66(1):386–393
Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26(4):435–439. doi:10.1038/82565
Rousset S, Emre Y, Join-Lambert O, Hurtaud C, Ricquier D, Cassard-Doulcier AM (2006) The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine 35(3–4):135–142. doi:10.1016/j.cyto.2006.07.012
Carrion J, Abengozar MA, Fernandez-Reyes M, Sanchez-Martin C, Rial E, Dominguez-Bernal G, Gonzalez-Barroso MM (2013) UCP2 deficiency helps to restrict the pathogenesis of experimental cutaneous and visceral leishmaniosis in mice. PLoS Negl Trop Dis 7(2):e2077. doi:10.1371/journal.pntd.0002077
Emre Y, Nubel T (2010) Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett 584(8):1437–1442. doi:10.1016/j.febslet.2010.03.014
Gonzalez-Barroso MM, Giurgea I, Bouillaud F, Anedda A, Bellanne-Chantelot C, Hubert L, de Keyzer Y, de Lonlay P, Ricquier D (2008) Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PloS One 3(12):e3850. doi:10.1371/journal.pone.0003850
Dienel GA (2012) Fueling and imaging brain activation. ASN Neuro. doi:10.1042/AN20120021
Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V (2014) Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014:472459. doi:10.1155/2014/472459
Yasuno K, Ando S, Misumi S, Makino S, Kulski JK, Muratake T, Kaneko N, Amagane H, Someya T, Inoko H, Suga H, Kanemoto K, Tamiya G (2007) Synergistic association of mitochondrial uncoupling protein (UCP) genes with schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet 144B(2):250–253. doi:10.1002/ajmg.b.30443
Campbell DA, Sundaramurthy D, Gordon D, Markham AF, Pieri LF (1999) Association between a marker in the UCP-2/UCP-3 gene cluster and genetic susceptibility to anorexia nervosa. Mol Psychiatr 4(1):68–70
Acknowledgments
The authors thank Barbara Sesé, Isabel Manso and María José Guillén for excellent technical support. This work was supported by Grants S2010/BMD-2402 and SAF2014-56929R to JS, S2010/BMD-2349 and SAF2014-57739-R to SC, and S2010/BMD-2402 and CSD2007-00020 to ER; and an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. LC has been the recipient of a Junta de Ampliación de Estudios-Consejo Superior de Investigaciones Científicas and CIBERER postdoctoral contracts. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Especial Issue: Tribute to Mary C McKenna.
Rights and permissions
About this article
Cite this article
Contreras, L., Rial, E., Cerdan, S. et al. Uncoupling Protein 2 (UCP2) Function in the Brain as Revealed by the Cerebral Metabolism of (1–13C)-Glucose. Neurochem Res 42, 108–114 (2017). https://doi.org/10.1007/s11064-016-1999-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1999-5