Abstract
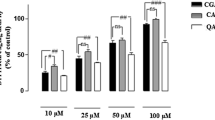
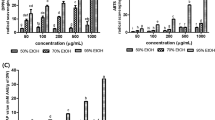
The effectiveness of chlorogenic acid and its main metabolites, caffeic and quinic acids, against oxidative stress was investigated. Resveratrol, another natural phenolic compound, was also tested for comparison. Rat cortical slices were incubated with 200 μM H2O2 for 1 h, and alterations in oxidative stress parameters, such as 2, 3, 5-triphenyltetrazolium chloride (TTC) staining and the production of both malondialdehyde (MDA) and reactive oxygen species (ROS), were assayed in the absence or presence of phenolic compounds. Additionally, the effectiveness of chlorogenic acid and other compounds on H2O2-induced increases in fluorescence intensities were also compared in slice-free incubation medium. Although quinic acid failed, chlorogenic and caffeic acids significantly ameliorated the H2O2-induced decline in TTC staining intensities. Although resveratrol also caused an increase in staining intensity, its effect was not dose-dependent; the high concentrations of resveratrol tested in the present study (10 and 100 μM) further lessened the staining of the slices. Additionally, all phenolic compounds significantly attenuated the H2O2-induced increases in MDA and ROS levels in cortical slices. When the IC50 values were compared to H2O2-induced alterations, chlorogenic acid was more potent than either its metabolites or resveratrol for all parameters studied under these experimental conditions. In slice-free experimental conditions, on the other hand, chlorogenic and caffeic acids significantly attenuated the fluorescence emission enhanced by H2O2 with a similar order of potency to that obtained in slice-containing physiological medium. These results indicate that chlorogenic acid is a more potent phenolic compound than resveratrol and its main metabolites caffeic and quinic acids against H2O2-induced alterations in oxidative stress parameters in rat cortical slices.







Similar content being viewed by others
References
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Halliwell B (2006) Oxidative stress and neurodegeneration: Where are we now? J Neurochem 97:1634–1658. doi:10.1111/j.1471-4159.2006.03907.x
Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111:163–169. doi:10.1172/JCI200317638
Thomas B (2009) Parkinson’s disease: from molecular pathways in disease to therapeutic approaches. Antioxid Redox Signal 11:2077–2082. doi:10.1089/ars.2009.2697
Zhu X, Lee HG, Perry G, Smith MA (2007) Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta Mol Basis Dis 1772:494–502. doi:10.1016/j.bbadis.2006.10.014
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184. doi:10.1016/j.lfs.2003.09.047
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203. doi:10.1016/j.foodchem.2005.07.042
Bagdas D, Etoz BC, Gul Z et al (2014) Chlorogenic acid enhances abdominal skin flap survival based on epigastric artery in nondiabetic and diabetic rats. Ann Plast Surg. doi:10.1097/SAP.0000000000000313
Bagdas D, Ozboluk HY, Cinkilic N, Gurun MS (2014) Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J Med Food 17:730–732. doi:10.1089/jmf.2013.2966
Bagdas D, Cam Etoz B, Inan Ozturkoglu S et al (2014) Effects of systemic chlorogenic acid on random-pattern dorsal skin flap survival in diabetic rats. Biol Pharm Bull 37:361–370
Bagdas D, Etoz BC, Gul Z et al (2015) In vivo systemic chlorogenic acid therapy under diabetic conditions: wound healing effects and cytotoxicity/genotoxicity profile. Food Chem Toxicol 81:54–61. doi:10.1016/j.fct.2015.04.001
Bagdas D, Gul NY, Topal A et al (2014) Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch Pharmacol 387:1101–1116. doi:10.1007/s00210-014-1034-9
Kanno Y, Watanabe R, Zempo H et al (2013) Chlorogenic acid attenuates ventricular remodeling after myocardial infarction in mice. Int Heart J 54:176–180
Li Y, Shen D, Tang X et al (2014) Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol Lett 226:257–263. doi:10.1016/j.toxlet.2014.02.016
Rispail N, Morris PM, Webb KJ (2005) Phenolic compounds: extraction and analysis. In: Márquez AJ, Stougaard J, Udvardi MK, Parniske M, Spaink HP, Saalbach G, Webb KJ, Chiurazzi M, Márquez AJ (eds) Lotus japonicus handbook. Springer, Netherlands, pp 349–354
Clifford MN (1999) Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J Sci Food Agric 79:362–372. doi:10.1002/(SICI)1097-0010(19990301)79:3<362:AID-JSFA256>3.0.CO;2-D
Sharma P (2011) Cinnamic acid derivatives: a new chapter of various pharmacological activities. J Chem Pharm Res 3:403–423
Avanesyan AA, Pashkov AN, Simonyan NA et al (2009) Antiradical activity of cinnamic acid derivatives. Pharm Chem J 43:249–250. doi:10.1007/s11094-009-0285-0
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49:472–481. doi:10.1002/mnfr.200500010
Yu C, Geun Shin Y, Chow A et al (2002) Human, rat, and mouse metabolism of resveratrol. Pharm Res 19:1907–1914. doi:10.1023/A:1021414129280
Azuma K, Ippoushi K, Nakayama M et al (2000) Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J Agric Food Chem 48:5496–5500. doi:10.1021/jf000483q
Gonthier M-P, Verny M-A, Besson C et al (2003) Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J Nutr 133:1853–1859
Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol 41:753–758
Wang S-H, Chen C-S, Huang S-H et al (2009) Hydrophilic ester-bearing chlorogenic acid binds to a novel domain to inhibit xanthine oxidase. Planta Med 75:1237–1240. doi:10.1055/s-0029-1185521
Gürsoy M, Büyükuysal RL (2008) Resveratrol protects rat striatal slices against anoxia-induced dopamine release. Neurochem Res 33:1838–1844. doi:10.1007/s11064-008-9645-5
Gürsoy M, Büyükuysal RL (2010) Mechanism of S100b release from rat cortical slices determined under basal and stimulated conditions. Neurochem Res 35:429–436. doi:10.1007/s11064-009-0075-9
Büyükuysal RL, Mete B (1999) Anoxia-induced dopamine release from rat striatal slices: involvement of reverse transport mechanism. J Neurochem 72:1507–1515
Preston E, Webster J (2000) Spectrophotometric measurement of experimental brain injury. J Neurosci Methods 94:187–192
Pilz J, Meineke I, Gleiter CH (2000) Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl 742:315–325
Demircan C, Gul Z, Buyukuysal RL (2014) High glutamate attenuates S100B and LDH outputs from rat cortical slices enhanced by either oxygen-glucose deprivation or menadione. Neurochem Res 39:1232–1244. doi:10.1007/s11064-014-1301-7
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Tallarida RJ, Murray RB (1987) Manual of pharmacologic calculations with computer programs, 2nd edn. Springer, New York
Feeney CJ, Frantseva MV, Carlen PL et al (2008) Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res 1198:1–15. doi:10.1016/j.brainres.2007.12.049
Cho S, Wood A, Bowlby MR (2007) Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol 5:19–33. doi:10.2174/157015907780077105
De Simoni A, Yu LMY (2006) Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc 1:1439–1445. doi:10.1038/nprot.2006.228
Schurr A, Rigor BM (eds) (1990) Cerebral ischemia and resuscitation. CRC Press Phillis JW, Boca Raton
Lein PJ, Barnhart CD, Pessah IN (2011) Acute hippocampal slice preparation and hippocampal slice cultures. Methods Mol Biol 758:115–134. doi:10.1007/978-1-61779-170-3_8
Cross AR, Jones OT (1991) Enzymic mechanisms of superoxide production. Biochim Biophys Acta 1057:281–298
Clapp PA, Davies MJ, French MS, Gilbert BC (1994) The bactericidal action of peroxides; an E.P.R. spin-trapping study. Free Radic Res 21:147–167
Hyslop PA, Hinshaw DB, Scraufstatter IU et al (1995) Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic Biol Med 19:31–37
Zhang J, Piantadosi CA (1992) Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest 90:1193–1199. doi:10.1172/JCI115980
Cohen G (1994) Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci 738:8–14
Desagher S, Glowinski J, Premont J (1996) Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci 16:2553–2562
Sokolova T, Gutterer JM, Hirrlinger J et al (2001) Catalase in astroglia-rich primary cultures from rat brain: immunocytochemical localization and inactivation during the disposal of hydrogen peroxide. Neurosci Lett 297:129–132
Avshalumov MV, Chen BT, Rice ME (2000) Mechanisms underlying H(2)O(2)-mediated inhibition of synaptic transmission in rat hippocampal slices. Brain Res 882:86–94
Pellmar TC, Neel KL, Lee KH (1989) Free radicals mediate peroxidative damage in guinea pig hippocampus in vitro. J Neurosci Res 24:437–444. doi:10.1002/jnr.490240314
Sah R, Schwartz-Bloom RD (1999) Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal neurons after oxidative stress. J Neurosci 19:9209–9217
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet (London, England) 344:721–724
Cuvelier M-E, Richard H, Berset C (1992) Comparison of the antioxidative activity of some acid-phenols: strucgure-activity relationship. Biosci Biotechnol Biochem 56:324–325. doi:10.1271/bbb.56.324
Chen JH, Ho C-T (1997) Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem 45:2374–2378. doi:10.1021/jf970055t
Milić BL, Djilas SM, Čanadanović-Brunet JM (1998) Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem 61:443–447. doi:10.1016/S0308-8146(97)00126-X
Medina I, Gallardo JM, Gonzalez MJ et al (2007) Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J Agric Food Chem 55:3889–3895. doi:10.1021/jf063498i
Marinova EM, Toneva A, Yanishlieva N (2009) Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502. doi:10.1016/j.foodchem.2008.11.045
Cheng J-C, Dai F, Zhou B et al (2007) Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure–activity relationship. Food Chem 104:132–139. doi:10.1016/j.foodchem.2006.11.012
Chu Y-F, Brown PH, Lyle BJ et al (2009) Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. J Agric Food Chem 57:9801–9808. doi:10.1021/jf902095z
Quincozes-Santos A, Bobermin LD, Latini A et al (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One 8:e64372. doi:10.1371/journal.pone.0064372
Sato Y, Itagaki S, Kurokawa T et al (2011) In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm 403:136–138. doi:10.1016/j.ijpharm.2010.09.035
Kim J, Lee S, Shim J et al (2012) Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem Int 60:466–474. doi:10.1016/j.neuint.2012.02.004
Oboh G, Agunloye OM, Akinyemi AJ et al (2012) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem Res 38:413–419. doi:10.1007/s11064-012-0935-6
Kwon S-H, Lee H-K, Kim J-A et al (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol 649:210–217. doi:10.1016/j.ejphar.2010.09.001
Heitman E, Ingram DK (2014) Cognitive and neuroprotective effects of chlorogenic acid. Nutr Neurosci doi:10.1179/1476830514Y.0000000146
Pero RW, Lund H, Leanderson T (2009) Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res 23:335–346. doi:10.1002/ptr.2628
Erdem MG, Cinkilic N, Vatan O et al (2012) Genotoxic and anti-genotoxic effects of vanillic acid against mitomycin C-induced genomic damage in human lymphocytes in vitro. Asian Pac J Cancer Prev 13:4993–4998
Galati G, Sabzevari O, Wilson JX, O’Brien PJ (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 177:91–104
Pasciu V, Posadino AM, Cossu A et al (2010) Akt downregulation by flavin oxidase-induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol Sci 114:101–112. doi:10.1093/toxsci/kfp301
Carru C, Pasciu V, Sotgia S et al (2011) The oxidative state of LDL is the major determinant of anti/prooxidant effect of coffee on cu catalysed peroxidation. Open Biochem J 5:1–8. doi:10.2174/1874091X01105010001
Gadacha W, Ben-Attia M, Bonnefont-Rousselot D et al (2009) Resveratrol opposite effects on rat tissue lipoperoxidation: pro-oxidant during day-time and antioxidant at night. Redox Rep 14:154–158. doi:10.1179/135100009X466131
Kalinich JF, Ramakrishnan N, McClain DE (1997) The antioxidant Trolox enhances the oxidation of 2′,7′-dichlorofluorescin to 2′,7′-dichlorofluorescein. Free Radic Res 26:37–47
Kalyaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H et al (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6
Acknowledgments
We would like to thank Uludag University Research Council for linguistic edition of the MS by American Journal Experts (AJE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gul, Z., Demircan, C., Bagdas, D. et al. Protective Effects of Chlorogenic Acid and its Metabolites on Hydrogen Peroxide-Induced Alterations in Rat Brain Slices: A Comparative Study with Resveratrol. Neurochem Res 41, 2075–2085 (2016). https://doi.org/10.1007/s11064-016-1919-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1919-8