Abstract
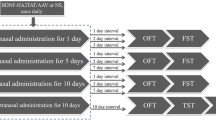
The present study was designed to construct a recombinant adeno-associated virus (rAAV) which can express NAP in the brain and examine whether this virus can produce antidepressant effects on C57 BL/6 mice that had been subjected to open field test and forced swimming test, via nose-to-brain pathway. When the recombinant plasmid pGEM-T Easy/NT4-NAP was digested by EcoRI, 297 bp fragments can be obtained and NT4-NAP sequence was consistent with the designed sequence confirmed by DNA sequencing. When the recombinant plasmid pSSCMV/NT4-NAP was digested by EcoRI, 297 bp fragments is visible. Immunohistochemical staining of fibroblasts revealed that expression of NAP was detected in NT4-NAP/AAV group. Intranasal delivery of NT4-NAP/AAV significantly reduced immobility time when the FST was performed after 1 day from the last administration. The effects observed in the FST could not be attributed to non-specific increases in activity since intranasal delivery of NT4-NAP/AAV did not alter the behavior of the mice during the open field test. The results indicated that a recombinant AAV vector which could express NAP in cells was successfully constructed and NAP may be a potential target for therapeutic action of antidepressant treatment.




Similar content being viewed by others
References
Kessler RC et al (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23):3095–3105
Rosenzweig-Lipson S et al (2007) Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther 113(1):134–153
Thase ME, Entsuah AR, Rudolph RL (2001) Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry 178:234–241
Bassan M et al (1999) Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 72(3):1283–1293
Magen I, Gozes I (2014) Davunetide: peptide therapeutic in neurological disorders. Curr Med Chem 21(23):2591–2598
Offen D et al (2000) Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson’s disease. Brain Res 854(1–2):257–262
Fleming SM et al (2011) A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions. Mol Cell Neurosci 46(3):597–606
Jouroukhin Y, Ostritsky R, Gozes I (2012) D-NAP prophylactic treatment in the SOD mutant mouse model of amyotrophic lateral sclerosis: review of discovery and treatment of tauopathy. J Mol Neurosci 48(3):597–602
Merenlender-Wagner A et al (2010) NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse—a microtubule-deficient model of schizophrenia. Peptides 31(7):1368–1373
Gozes I et al (2002) NAP accelerates the performance of normal rats in the water maze. J Mol Neurosci 19(1–2):167–170
Morimoto BH et al (2009) Davunetide pharmacokinetics and distribution to brain after intravenous or intranasal administration to rat. Chim Oggi 27(2):16–20
Serlin Y et al (2015) Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol 38:2–6
Holman BL (1972) The blood brain barrier: anatomy and physiology. Prog Nucl Med 1:236–248
Stieger K et al (2009) Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther 17(3):516–523
Morimoto BH et al (2013) Davunetide: a review of safety and efficacy data with a focus on neurodegenerative diseases. Expert Rev Clin Pharmacol 6(5):483–502
Gozes I et al (2005) NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP). CNS Drug Rev 11(4):353–368
Vasil Yeva NA, Murzina GB, Pivovarov AS (2015) Habituation-like decrease of acetylcholine-induced inward current in helix command neurons: role of microtubule motor proteins. Cell Mol Neurobiol 35(5):703–712
Wong GT, Chang RC, Law AC (2013) A breach in the scaffold: the possible role of cytoskeleton dysfunction in the pathogenesis of major depression. Ageing Res Rev 12(1):67–75
Lalatsa A, Schatzlein AG, Uchegbu IF (2014) Strategies to deliver peptide drugs to the brain. Mol Pharm 11(4):1081–1093
Graff CL, Pollack GM (2005) Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci 94(6):1187–1195
Mistry A, Stolnik S, Illum L (2009) Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 379(1):146–157
Vaka SRK et al (2012) Delivery of brain-derived neurotrophic factor via nose-to-brain pathway. Pharm Res 29(2):441–447
Dufes C et al (2003) Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int J Pharm 255(1–2):87–97
Yu H, Kim K (2009) Direct nose-to-brain transfer of a growth hormone releasing neuropeptide, hexarelin after intranasal administration to rabbits. Int J Pharm 378(1–2):73–79
Mittal D et al (2014) Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv 21(2):75–86
Zheng G et al (2011) Adeno-associated viral vector-mediated expression of NT4-ADNF-9 fusion gene protects against aminoglycoside-induced auditory hair cell loss in vitro. Acta Otolaryngol 131(2):136–141
Vale RD (2003) The molecular motor toolbox for intracellular transport. Cell 112(4):467–480
Magen I, Gozes I (2013) Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). Neuropeptides 47(6):489–495
Divinski I, Mittelman L, Gozes I (2004) A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem 279(27):28531–28538
Jouroukhin Y et al (2013) NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiol Dis 56:79–94
Idan-Feldman A, Ostritsky R, Gozes I (2012) Tau and caspase 3 as targets for neuroprotection. Int J Alzheimer’s Dis 2012:1–8
Oz S et al (2014) The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry 19(10):1115–1124
Harrod SB, Van Horn ML (2009) Sex differences in tolerance to the locomotor depressant effects of lobeline in periadolescent rats. Pharmacol Biochem Behav 94(2):296–304
Mill J, Petronis A (2007) Molecular studies of major depressive disorder: the epigenetic perspective. Mol Psychiatry 12(9):799–814
Acknowledgments
The project was supported by the National Science Foundation of China (No. 81171256 to Xian-Cang Ma, No. 81271487 to Cheng-ge Gao and No. 81270416 to Xiao-Ling Zhang).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, XC., Chu, Z., Zhang, XL. et al. Intranasal Delivery of Recombinant NT4-NAP/AAV Exerts Potential Antidepressant Effect. Neurochem Res 41, 1375–1380 (2016). https://doi.org/10.1007/s11064-016-1841-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1841-0