Abstract
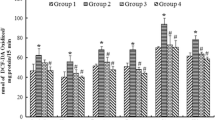
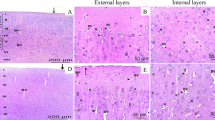
Our goal was to delineate the mechanisms of selenite-induced oxidative stress in neonatal rats and investigate the potential of blueberry leaf polyphenols to counteract the induced stress. Vaccinium corymbosum leaf decoction (BLD) was analyzed by UPLC-MS and LC-DAD, along with its in vitro antioxidant activity (DPPH radical scavenging, FRAP, ferrous chelation). Newborn suckling Wistar rats were randomly divided into three groups: ‘Se’ and ‘SeBLD’ received 20 μmol Na2SeO3/kg BW subcutaneously (PN day 10); ‘SeBLD’ received 100 mg dry BLD/kg BW intraperitoneally (PN11 and 12) and Group ‘C’ received normal saline. Βiochemical analysis revealed tissue-specific effects of selenite. Brain as a whole was more resistant to selenite toxicity in comparison to liver; midbrain and cerebellum were in general not affected, but cortex was moderately disturbed. Liver lipid peroxidation, GSH, SOD, CAT, GPx were significantly affected, whereas proteolytic activity was not. BLD, which is rich in chlorogenic acid and flavonols (especially quercetin derivatives), exerted significant antioxidant protective effects in all regions. In conclusion, we provide for the first time an insight to the neonatal rat cerebral and liver redox response against a toxic selenite dose and blueberry leaf polyphenols.





Similar content being viewed by others
References
Postgate JR (1952) Competitive and noncompetitive inhibitors of bacterial sulphate reduction. J Gen Microbiol 6:128–142
Ostadalova I, Babicky A, Obenberger J (1978) Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia 34:222–223
Shearer TR, McCormack DW, DeSart DJ, Britton JL, Lopez MT (1980) Histological evaluation of selenium induced cataracts. Exp Eye Res 31:327–333
Bunce GE, Hess JL (1981) Biochemical changes associated with selenite-induced cataract in the rat. Exp Eye Res 33:505–514
Ostadalova I, Babicky A, Obenberger J (1979) Cataractogenic and lethal effect of selenite in rats during postnatal ontogenesis. Physiol Bohemoslov 28:393–397
Kramer GF, Ames BN (1988) Mechanisms of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat Res 201:169–180
Newland MC, Hoffman DJ, Heath JC, Donlin WD (2013) Response inhibition is impaired by developmental methylmercury exposure: acquisition of low-rate lever-pressing. Behav Brain Res 253:196–205
Schweizer U, Brauer AU, Kohrle J, Nitsch R, Savaskan NE (2004) Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev 45:164–178
Gomez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9:568–578
Spencer JP (2009) Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr 4:243–250
Williams RJ, Spencer JP (2012) Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 52:35–45
Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN (2009) Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res 198:352–358
Papandreou MA, Tsachaki M, Efthimiopoulos S, Klimis-Zacas D, Margarity M, Lamari FN (2012) Cell-line specific protection by berry polyphenols against hydrogen peroxide challenge and lack of effect on metabolism of amyloid precursor protein. Phytother Res 26:956–963
Tsao R, Yang R (2003) Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography. J Chromatogr A 1018:29–40
Wang T, Jónsdóttir R, Ólafsdóttir G (2009) Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–248
Li WJ, Cheng XL, Liu J, Lin RC, Wang GL, Du SS, Liu ZL (2012) Phenolic compounds and antioxidant activities of Liriope muscari. Molecules 17:1797–1808
Katalinic V, Milos M, Kulisic T, Jukic M (2006) Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem 94:550–557
Ren Z, He C, Fan Y, Guo L, Si H, Wang Y, Shi Z, Zhang H (2014) Immuno-enhancement effects of ethanol extract from Cyrtomium macrophyllum (Makino) Tagawa on cyclophosphamide-induced immunosuppression in BALB/c mice. J Ethnopharmacol 155:769–775
Pallares V, Fernandez-Iglesias A, Cedo L, Castell-Auvi A, Pinent M, Ardevol A, Salvado MJ, Garcia-Vallve S, Blay M (2013) Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic Biol Med 60:107–114
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Grotto D, Santa Maria LD, Boeira S, Valentini J, Charao MF, Moro AM, Nascimento PC, Pomblum VJ, Garcia SC (2007) Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal 43:619–624
Jentzsch AM, Bachmann H, Furst P, Biesalski HK (1996) Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 20:251–256
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Makri OE, Ferlemi AV, Lamari FN, Georgakopoulos CD (2013) Saffron administration prevents selenite-induced cataractogenesis. Mol Vis 19:1188–1197
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sasaki T, Kikuchi T, Yumoto N, Yoshimura N, Murachi T (1984) Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem 259:12489–12494
Khan JY, Black SM (2003) Developmental changes in murine brain antioxidant enzymes. Pediatr Res 54:77–82
Shivakumar BR, Anandatheerthavarada HK, Ravindranath V (1991) Free radical scavenging systems in developing rat brain. Int J Dev Neurosci 9:181–185
Danscher G (1982) Exogenous selenium in the brain. A histochemical technique for light and electron microscopical localization of catalytic selenium bonds. Histochemistry 76:281–293
Downes N, Mullins P (2013) The Development of myelin in the brain of the Juvenile rat. Toxicol Pathol 42:913–922
Sauvageot CM, Stiles CD (2002) Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol 12:244–249
Camins A, Verdaguer E, Folch J, Pallas M (2006) Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev 12:135–148
Hamakubo T, Kannagi R, Murachi T, Matus A (1986) Distribution of calpains I and II in rat brain. J Neurosci 6:3103–3111
Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: A mechanism of “pathological apoptosis”? J Biol Chem 276:10191–10198
Ostadalova I, Babicky A, Kopoldova J (1988) Selenium metabolism in rats after administration of toxic doses of selenite. Physiol Bohemoslov 37:159–164
Anundi I, Stahl A, Hogberg J (1984) Effects of selenite on O2 consumption, glutathione oxidation and NADPH levels in isolated hepatocytes and the role of redox changes in selenite toxicity. Chem Biol Interact 50:277–288
Stewart MS, Spallholz JE, Neldner KH, Pence BC (1999) Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic Biol Med 26:42–48
Gavrilova V, Kajdzanoska M, Gjamovski V, Stefova M (2011) Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J Agric Food Chem 59:4009–4018
Oszmianski J, Wojdylo A, Gorzelany J, Kapusta I (2011) Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J Agric Food Chem 59:12830–12835
Harris CS, Burt AJ, Saleem A, Le PM, Martineau LC, Haddad PS, Bennett SA, Arnason JT (2007) A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem Anal 18:161–169
Matsuo Y, Fujita Y, Ohnishi S, Tanaka T, Hirabaru H, Kai T, Sakaida H, Nishizono S, Kouno I (2010) Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and A-type linkages. Food Chem 121:1073–1079
Piljac-Zegarac J, Belscak A, Piljac A (2009) Antioxidant capacity and polyphenolic content of blueberry (Vaccinium corymbosum L.) leaf infusions. J Med Food 12:608–614
Loh G, Romo-Vaquero M, Selma M-V, Larrosa M, Obiol M, García-Villalba R, González-Barrio R, Issaly N, Flanagan J, Roller M, Tomás-Barberán FA, García-Conesa M-T (2014) A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits β-glucosidase activity, altering fiber and short chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 9:e94687
Jacobs H, Moalin M, van Gisbergen MW, Bast A, van der Vijgh WJ, Haenen GR (2011) An essential difference in the reactivity of the glutathione adducts of the structurally closely related flavonoids monoHER and quercetin. Free Radic Biol Med 51:2118–2123
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative medicine and cellular longevity 2012:1–16
Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y, Terao J (2011) Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med 51:1329–1336
Paulke A, Eckert GP, Schubert-Zsilavecz M, Wurglics M (2012) Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie 67:991–996
Schaffer S, Halliwell B (2012) Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr 7:99–109
Wu K, Wang ZZ, Liu D, Qi XR (2014) Pharmacokinetics, brain distribution, release and blood-brain barrier transport of Shunaoxin pills. J Ethnopharmacol 151:1133–1140
Dogan Z, Kocahan S, Erdemli E, Kose E, Yilmaz I, Ekincioglu Z, Ekinci N, Turkoz Y (2014) Effect of chemotherapy exposure prior to pregnancy on fetal brain tissue and the potential protective role of quercetin. Cytotechnology. doi:10.1007/s10616-014-9742-z
Lakroun Z, Kebieche M, Lahouel A, Zama D, Desor F, Soulimani R (2015) Oxidative stress and brain mitochondria swelling induced by endosulfan and protective role of quercetin in rat. Environ Sci Pollut Res Int 22:7776–7781
Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S (2008) Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr Aging Sci 1:169–174
Hamza RZ, El-Shenawy NS, Ismail HA (2015) Protective effects of blackberry and quercetin on sodium fluoride-induced oxidative stress and histological changes in the hepatic, renal, testis and brain tissue of male rat. J Basic Clin Physiol Pharmacol 26:237–251
Jeong HR, Jo YN, Jeong JH, Kim HJ, Kim MJ, Heo HJ (2013) Blueberry (Vaccinium virgatum) leaf extracts protect against Abeta-induced cytotoxicity and cognitive impairment. J Med Food 16:968–976
Acknowledgments
The study was supported by ‘K. Karatheodoris’ Grant No. C913 from the Research Committee, University of Patras, Greece. The authors kindly thank the Greek Cooperative ‘Biodrama’, East Macedonia, Greece, for the offer of dried highbush blueberry leaves.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferlemi, AV., Mermigki, P.G., Makri, O.E. et al. Cerebral Area Differential Redox Response of Neonatal Rats to Selenite-Induced Oxidative Stress and to Concurrent Administration of Highbush Blueberry Leaf Polyphenols. Neurochem Res 40, 2280–2292 (2015). https://doi.org/10.1007/s11064-015-1718-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1718-7