Abstract
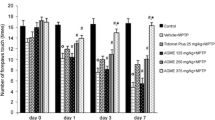
Numerous studies indicating that natural plant sources and their active phytochemicals offer protection to the pathological processes related to the development of neurogenerative diseases including Parkinson’s disease (PD). In the present study, the neuro protective efficacy of dietary supplementation of walnut (6 %) for 28 days was examined in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (i.p., 20 mg/kg body weight/day) for last four consecutive days. MPTP injection diminished the levels of GSH, dopamine and metabolites along with decreased activities of GPx and mitochondrial complex I. Further, the levels of TBARS and enzymatic antioxidants such as SOD and catalase, MAO-B activities were enhanced by MPTP treatment. Behavioral deficits and lowered TH expression are also proved MPTP induced neurotoxicity. Dietary supplementation of walnut attenuated MPTP-induced impairment in PD mice might be by its MAO-B inhibitory, antioxidant and mitochondrial protective actions. To find out the exact mechanism of action walnut on PD mice warrants further extensive studies.









Similar content being viewed by others
Change history
18 October 2019
The original version of this article unfortunately contains an error in Fig. 2a (4th image for walnut). This has been corrected by publishing this erratum.
References
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Schmidt N, Ferger B (2001) Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm 108:1263–1282
Hardman WE, Ion G (2008) Suppression of implanted MDA-MB231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer 60:666–674
Shimoda H, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T (2008) Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J Agric Food Chem 56:4444–4449
Jiang R, Manson JE, Stamfer MJ, Liu S, Willett WC, Hu FB (2002) Nut and pea nut butter consumption and risk of type 2 diabetes in women. JAMA 288:2554–2560
Gandev S (2007) Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria. Bulg J Agric Sci 13:683–689
Iwamoto M, Sato M, Kono M, Hirooka Y, Saka K, Takeshita A, Imaizumi K (2000) Walnuts lower serum cholesterol in Japanese men and women. J Nutr 130:171–176
Ros E, Nnez I, Perez-Heras A, Merce S, Gilabert R, Casals E, Deulofeu R (2004) Walnut diet improves endothelial functions in hypercholesterolemic subject. Circulation 109:1609–1614
Spaccarotella KJ, Kris-Etherton PM, Stone WL, Bagshaw DM, Fishell VK, West SG, Lawrence FR, Hartman TJ (2008) The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J 7:13
Muthaiyah B, Essa MM, Lee M, Chauhan V, Kaur K, Chauhan A (2014) Dietary supplementation of walnut improves memory deficits and learning skills in mouse model of Alzheimer’s disease. J Alzheimers Dis 42(4):1397–1405. doi:10.3233/JAD-140675
Crews C, Hough P, Godward J, Brereton P, Lees M, Guiet S, Winkelmann W (2005) Study of the main constituents of some authentic walnut oils. J Agric Food Chem 53:4853–4860
Braidy N, Subash S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, Al-Senawi H, Alobaidy AAR, Lakhtakia R, Guillemin GJ (2013) Neuroprotective effects of a variety of pomegranate juice extracts (PJE) against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid Med Cell Longev. doi:10.1155/2013/685909
Maraldi M, Vauzour D, Angeloni C (2014) Dietary polyphenols and their effects on cell biochemistry and pathophysiology. Oxid Med Cell Longev 2014:576363. doi:10.1155/2014/576363
Muthaiyah B, Essa MM, Chauhan C, Chauhan V (2011) Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res 36:2096–2103
Park G, Kim HG, Hong SP, Kim SY, Oh MS (2014) Walnuts (seeds of Juglandis sinensis L.) protect human epidermal keratinocytes against UVB-induced mitochondria-mediated apoptosis through upregulation of ROS elimination pathways. Skin Pharmacol Physiol 27(3):132–140. doi:10.1159/000354917 Epub 2014 Jan 11
International Union of Pure and Applied Chemistry (IUPAC) (1992) Standard methods for the analysis of oils, fats and derivatives (1st Suppl. to 7th ed., Enlarged ed.). Blackwell Scientific Publications, Oxford (Methods 2201, 2205, 2432, 2301, 2302, 2324, and 2507)
Calvo P, Castaño ÁL, Hernández MT, González-Gómez D (2011) Effects of microcapsule constitution into the quality of microencapsulated walnut oil. Eur J Lipid Sci Technol 113:1273–1280
Burkhardt S, Xian Tan D, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J Agric Food Chem 49:4898–4902
Lima VLAG, Mélo EA, Maciel MIS, Prazeres FG, Musser RS, Lima DES (2005) Total phenolic and carotenoid content in acerola genotypes harvested at three ripening stages. Food Chem 90:565–568
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR (2009) Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res 16:127–139
Rojas P, Rios C (1993) Increased striatal lipid peroxidation after inter cerbro ventricular MPP+ administration to mice. Pharmacol Toxicol 72:364–368
Mohanasundari M, Srinivasan MS, Sethupathy S, Sabesan M (2006) Enhanced neuroprotective effect by combination of bromocriptine and Hypericumperforatum extract against MPTP-induced neurotoxicity in mice. J Neurol Sci 249:140–144
Rajasankar S, Manivasagam T, Surendran S (2009) Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci Lett 454:11–15
Kavitha M, Nataraj J, Essa MM, Memon MA, Manivasagam T (2013) Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem Biol Interact 206:239–247
Bhattacharya A, Ghosal S, Bhattacharya SK (2001) Antioxidant effect of Withania somnifera glycol withanolides in chronic foot-shock stress induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol 74:1–16
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Yamamoto Y, Takahashi K (1993) Glutathione peroxidase isolated from plasma reduces phospholipid hydroperoxide. Arch Biochem Biophys 305:541–545
Oberley LW, Spitz DR (1984) Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol 105:457–464
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73:1127–1137
King TE, Howard RL (1967) Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10:275–294
Patki G, Che Y, Lau Y (2009) Mitochondrial dysfunction in the striatum of aged chronic mouse model of Parkinson’s disease. Front Aging Neurosci 1:1–9
Davis L, Stonehouse W, Loots DT, Mukuddem-Petersen J, van der Westhuizen F, Hanekom SJ, Jerling JC (2007) The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr 46:155–164
Li D, Saldeen T, Romeo F, Mehta JL (1999) Relative effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation and superoxide dismutase and nitric oxide synthase activity and protein expression in rats. J Cardiovasc Pharmacol Ther 4:219–226
Kornsteiner M, Wagner KH, Elmadfa I (2006) Tocopherol and total phenolics in 10 different nut types. Food Chem 98:381–387
Reiter RJ, Manchester LC, Dun-xian Tan MD (2005) Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21:920–924
Ji L, Chauhan A, Wegiel J, Essa MM, Chauhan V (2009) Gelsolin is proteolytically cleaved in the brains of individuals with Alzheimer’s disease. J Alzheimers Dis 18(1):105–111
RajaSankar S, Manivasagam T, Surendran S (2009) Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci Lett 454:11–15
Tatton NA, Kish SJ (1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77:1037–1048
Ortiz GG, Pacheco-Moisés FP, Gómez-Rodríguez VM, González-Renovato ED, Torres-Sánchez ED, Ramírez-Anguiano AC (2013) Fish oil, melatonin and vitamin E attenuates midbrain cyclooxygenase-2 activity and oxidative stress after the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Metab Brain Dis 28:705–709
Khan HA (2010) Selenium partially reverses the depletion of striatal dopamine and its metabolites in MPTP-treated C57BL mice. Neurochem Int 57:489–491
Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP (2002) Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem 80:101–110
Bousquet M, Gibrat C, Saint-Pierre M, Julien C, Calon F, Cicchetti F (2009) Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model. Prog Neuropsychopharmacol Biol Psychiatry 33:1401–1408
Park G, Park YJ, Yang HO, Oh MS (2013) Ropinirole protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice via anti-apoptotic mechanism. Pharmacol Biochem Behav 104:163–168
Sedelis M, Schwarting RK, Huston JP (2001) Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res 125:109–125
Anandhan A, Tamilselvam K, Radhiga T, Rao S, Essa MM, Manivasagam T (2012) Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res 1433:104–113
Fredriksson A, Archer T (1994) MPTP-induced behavioural and biochemical deficits: a parametric analysis. J Neural Transm Park Dis Dement Sect 7:123–132
Heikkila RE, Hess A, Duvoisin RC (1984) Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 224:1451–1453
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G (2008) Mitochondrial dysfunction in rat brain with aging involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53:126–131
Schapira AHV, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ, reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145
Muralikrishnan D, Mohanakumar KP (1998) Neuroprtotection by bromocriptine against 1-methy1 4-pheny1 1,2,3,6 tetrahydrophyridine induced neurotoxicity in mice. FASEB J 12:905–912
Chen X, Zhou Y, Chen Y, Zhu Y, Fang F, Chen L (2005) Ginsenoside Rg1 reduces MPTP induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin 26:56–62
Rajeswari A (2006) Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Eur Rev Med Pharmacol Sci 10:157–161
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR Jr, Blomhoff R (2006) Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr 84:95–135
Mishra N, Dubey A, Mishra R, Barik N (2010) Study on antioxidant activity of common dry fruits. Food Chem Toxicol 48:3316–3320
Haddad EH, Gaban-Chong N, Oda K, Sabaté J (2014) Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J 10(13):4
Asadi-Shekaari M, Kalantaripour TP, Nejad FA, Namazian E, Eslami A (2012) The anticonvulsant and neuroprotective effects of walnuts on the neurons of rat brain cortex. Avicenna J Med Biotechnol 4:155–158
Carey AN, Fisher DR, Joseph JA, Shukitt-Hale dB (2013) The ability of walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutr Neurosci 16:13–20
Acknowledgments
The project supported by an internal Grant (IG/AGR/FOOD/14/01) also highly acknowledged. The project was also supported by the Research Council; Oman (Grant No. RC/AGR/FOOD/11/01) is gratefully acknowledged.
Conflict of interest
All the authors declaring no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Essa, M.M., Subash, S., Dhanalakshmi, C. et al. Dietary Supplementation of Walnut Partially Reverses 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Induced Neurodegeneration in a Mouse Model of Parkinson’s Disease. Neurochem Res 40, 1283–1293 (2015). https://doi.org/10.1007/s11064-015-1593-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1593-2