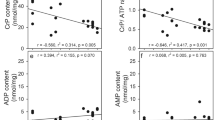
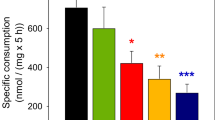
Abstract
Mammalian AMP-activated protein kinase (AMPK) functions as a metabolic switch. It is composed of 3 different subunits and its activation depends on phosphorylation of a threonine residue (Thr172) in the α-subunit. This phosphorylation can be brought about by 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) which in the cells is converted to a monophosphorylated nucleotide mimicking the effect of AMP. We show that the preparation of cultured astrocytes used for metabolic studies expresses AMPK, which could be phosphorylated by exposure of the cells to AICAR. The effect of AMPK activation on glutamate metabolism in astrocytes was studied using primary cultures of these cells from mouse cerebral cortex during incubation in media containing 2.5 mM glucose and 100 µM [U-13C]glutamate. The metabolism of glutamate including a detailed analysis of its metabolic pathways involving the tricarboxylic acid (TCA) cycle was studied using high-performance liquid chromatography analysis supplemented with gas chromatography–mass spectrometry technology. It was found that AMPK activation had profound effects on the pathways involved in glutamate metabolism since the entrance of the glutamate carbon skeleton into the TCA cycle was reduced. On the other hand, glutamate uptake into the astrocytes as well as its conversion to glutamine catalyzed by glutamine synthetase was not affected by AMPK activation. Interestingly, synthesis and release of citrate, which are hallmarks of astrocytic function, were affected by a reduction of the flux of glutamate derived carbon through the malic enzyme and pyruvate carboxylase catalyzed reactions. Finally, it was found that in the presence of glutamate as an additional substrate, glucose metabolism monitored by the use of tritiated deoxyglucose was unaffected by AMPK activation. Accordingly, the effects of AMPK activation appeared to be specific for certain key processes involved in glutamate metabolism.




Similar content being viewed by others
Abbreviations
- AAT:
-
Aspartate aminotransferase
- AICAR:
-
5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside
- ALAT:
-
Alanine aminotransferase
- AMPK:
-
AMP activated protein kinase
- BCA:
-
Bicinchoninic acid
- BSA:
-
Bovine serum albumin
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FCS:
-
Foetal calf serum
- GDH:
-
Glutamate dehydrogenase
- GC–MS:
-
Gas chromatography–mass spectrometry
- HPLC:
-
High-performance liquid chromatography
- LDH:
-
Lactate dehydrogenase
- ME:
-
Malic enzyme
- OPA:
-
o-Phthaldialdehyde
- PBS:
-
Phosphate buffered saline
- PC:
-
Pyruvate carboxylase
- PDH:
-
Pyruvate dehydrogenase
- TCA:
-
Tricarboxylic acid
References
Hertz L, Dienel GA (2002) Energy metabolism in the brain. Int Rev Neurobiol 51:1–102
Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC (2014) Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 11:13–30. doi:10.1007/978-3-319-08894-5_2
McKenna MC (2013) Glutamate pays its own way in astrocytes. Front Endocrinol 4:191. doi:10.3389/fendo.2013.00191
Dienel GA (2013) Astrocytic energetics during excitatory neurotransmission: what are contributions of glutamate oxidation and glycolysis? Neurochem Int 63(4):244–258. doi:10.1016/j.neuint.2013.06.015
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277(1 Pt 1):E1–10
Mitchelhill KI, Stapleton D, Gao G, House C, Michell B, Katsis F, Witters LA, Kemp BE (1994) Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem 269(4):2361–2364
Stapleton D, Gao G, Michell BJ, Widmer J, Mitchelhill K, Teh T, House CM, Witters LA, Kemp BE (1994) Mammalian 5’-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem 269(47):29343–29346
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262. doi:10.1038/nrm3311
Salt IP, Johnson G, Ashcroft SJ, Hardie DG (1998) AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J 335(Pt 3):533–539
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10(20):1247–1255
Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA (1999) Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 24(1):22–25
Moore F, Weekes J, Hardie DG (1991) Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem/FEBS 199(3):691–697
Yu AC, Drejer J, Hertz L, Schousboe A (1983) Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 41(5):1484–1487
Patel MS (1974) The relative significance of CO2-fixing enzymes in the metabolism of rat brain. J Neurochem 22(5):717–724
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161(2):303–310
Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS (2006) Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab 26(10):1285–1297. doi:10.1038/sj.jcbfm.9600281
Cruz F, Scott SR, Barroso I, Santisteban P, Cerdan S (1998) Ontogeny and cellular localization of the pyruvate recycling system in rat brain. J Neurochem 70(6):2613–2619
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229(2):558–565
Zhang L, Frederich M, He H, Balschi JA (2006) Relationship between 5-aminoimidazole-4-carboxamide-ribotide and AMP-activated protein kinase activity in the perfused mouse heart. Am J Physiol Heart Circ Physiol 290(3):H1235–H1243. doi:10.1152/ajpheart.00906.2005
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28(5):897–916
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2001) Multiple compartments with different metabolic characteristics are involved in biosynthesis of intracellular and released glutamine and citrate in astrocytes. Glia 35(3):246–252
Sonnewald U, Westergaard N, Krane J, Unsgard G, Petersen SB, Schousboe A (1991) First direct demonstration of preferential release of citrate from astrocytes using [13C]NMR spectroscopy of cultured neurons and astrocytes. Neurosci Lett 128(2):235–239
Hertz L, Juurlink B, Hertz E, Fosmark H (1989) Preparation of primary cultures of mouse (rat) astrocytes. In: Shahar A, De Vellis J, Haber B (eds) A dissection and tissue culture manual of the nervous system. Liss Inc, New York, pp 105–108
Walls A, Bak L, Sonnewald U, Schousboe A, Waagepetersen H (2014) Metabolic mapping of astrocytes and neurons in culture using stable isotopes and gas chromatography–mass spectrometry (GC-MS). In: Hirrlinger J, Waagepetersen HS (eds) Brain energy metabolism, vol 90. Neuromethods. Springer New York, pp 73–105. doi:10.1007/978-1-4939-1059-5_4
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imagej: 25 years of image analysis. Nat Methods 9(7):671–675
Mawhinney TP, Robinett RS, Atalay A, Madson MA (1986) Analysis of amino acids as their tert.-butyldimethylsilyl derivatives by gas-liquid chromatography and mass spectrometry. J Chromatogr 358(1):231–242
Biemann K (1962) Organic chemistry applications. Mass spectrometry. McGraw, New York, pp 223–227
Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. (2009) AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 109 (Suppl 1):17–23.
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48(8):1667–1671
Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW (2009) Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci 29(9):2997–3008. doi:10.1523/JNEUROSCI.0354-09.2009
Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27(2):219–249. doi:10.1038/sj.jcbfm.9600343
Almeida A, Moncada S, Bolanos JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6(1):45–51. doi:10.1038/ncb1080
Barros LF, Bittner CX, Loaiza A, Ruminot I, Larenas V, Moldenhauer H, Oyarzun C, Alvarez M (2009) Kinetic validation of 6-NBDG as a probe for the glucose transporter GLUT1 in astrocytes. J Neurochem 109(Suppl 1):94–100. doi:10.1111/j.1471-4159.2009.05885.x
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27(11):1766–1791. doi:10.1038/sj.jcbfm.9600521
Lee CT, Ussher JR, Mohammad A, Lam A, Lopaschuk GD (2014) 5′-AMP-activated protein kinase increases glucose uptake independent of GLUT4 translocation in cardiac myocytes. Can J Physiol Pharmacol 92(4):307–314. doi:10.1139/cjpp-2013-0107
Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L (1993) Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci 15(3–5):359–366
Westergaard N, Drejer J, Schousboe A, Sonnewald U (1996) Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia 17(2):160–168. doi:10.1002/(SICI)1098-1136(199606)17:2<160:AID-GLIA7>3.0.CO;2-6
Yu AC, Schousboe A, Hertz L (1982) Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39(4):954–960
Dawson NJ, Storey KB (2012) An enzymatic bridge between carbohydrate and amino acid metabolism: regulation of glutamate dehydrogenase by reversible phosphorylation in a severe hypoxia-tolerant crayfish. J Comp Physiol [B] 182(3):331–340. doi:10.1007/s00360-011-0629-4
Sonnewald U (2014) Glutamate synthesis has to be matched by its degradation: where do all the carbons go? J Neurochem 131(4):399–406. doi:10.1111/jnc.12812
Zwingmann C, Richter-Landsberg C, Leibfritz D (2001) 13C isotopomer analysis of glucose and alanine metabolism reveals cytosolic pyruvate compartmentation as part of energy metabolism in astrocytes. Glia 34(3):200–212
Westergaard N, Sonnewald U, Unsgard G, Peng L, Hertz L, Schousboe A (1994) Uptake, release, and metabolism of citrate in neurons and astrocytes in primary cultures. J Neurochem 62(5):1727–1733
Westergaard N, Banke T, Wahl P, Sonnewald U, Schousboe A (1995) Citrate modulates the regulation by Zn2+ of N-methyl-d-aspartate receptor-mediated channel current and neurotransmitter release. Proc Natl Acad Sci USA 92(8):3367–3370
Kaufman EE, Driscoll BF (1992) Carbon dioxide fixation in neuronal and astroglial cells in culture. J Neurochem 58(1):258–262
Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25(18):1895–1908. doi:10.1101/gad.17420111
Acknowledgments
This work was supported by the Novo Nordisk Foundation (2293). Heidi Nielsen and Anna Hansen are acknowledged for their excellent technical assistance. Stud. pharm. Omran Abdel Rahman is acknowledged for contributing to GC–MS analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Dr. Gerald Dienel.
Rights and permissions
About this article
Cite this article
Voss, C.M., Pajęcka, K., Stridh, M.H. et al. AMPK Activation Affects Glutamate Metabolism in Astrocytes. Neurochem Res 40, 2431–2442 (2015). https://doi.org/10.1007/s11064-015-1558-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1558-5