Abstract
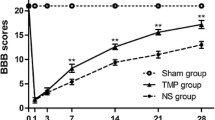
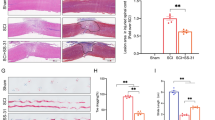
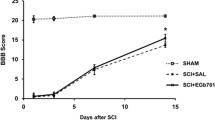
Many studies have illustrated that much of the post-traumatic degeneration of the spinal cord cells is caused by the secondary mechanism. The aim of this study is to evaluate the effect of the anti-inflammatory property of valproic acid (VPA) on injured spinal cords (SC). The rats with the contused SC received intraperitoneal single injection of VPA (150, 200, 300, 400 or 500 mg/kg) at 2, 6, 12 and 24 h post-injury. Basso–Beattie–Bresnahan (BBB) test and H-reflex evaluated the functional outcome for 12 weeks. The SC were investigated 3 months post-injury using morphometry and glial fibrillary acid protein (GFAP) expression. Reduction in cavitation, H/M ratio, BBB scores and GFAP expression in the treatment groups were significantly more than that of the untreated one (P < 0.05). The optimal improvement in the condition of the contused rats was in the ones treated at the acute phase of injury with 300 mg/kg of VPA at 12 h post-injury, they had the highest increase in BBB score and decrease in astrogliosis and axonal loss. We conclude that treating the contused rats with 300 mg/kg of VPA at 12 h post-injury improves the functional outcome and reduces the traumatized SC gliosis.








Similar content being viewed by others
References
Giannini MJ, Bergmark B, Kreshover S, Elias E, Plummer C, O’Keefe E (2010) Understanding suicide and disability through three major disabling conditions: intellectual disability, spinal cord injury, and multiple sclerosis. Disabil Health J 3(2):74–78. doi:10.1016/j.dhjo.2009.09.001
Cadotte DW, Fehlings MG (2011) Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 469(3):732–741. doi:10.1007/s11999-010-1674-0
Wu J, Kharebava G, Piao C, Stoica BA, Dinizo M, Sabirzhanov B, Hanscom M, Guanciale K, Faden AI (2012) Inhibition of E2F1/CDK1 pathway attenuates neuronal apoptosis in vitro and confers neuroprotection after spinal cord injury in vivo. PLoS One 7(7):e42129. doi:10.1371/journal.pone.0042129
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG (2008) Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 25(5):E2. doi:10.3171/foc.2008.25.11.e2
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399. doi:10.1038/nrn3053
Hawthorne AL, Popovich PG (2011) Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics 8(2):252–261. doi:10.1007/s13311-011-0032-6
Spitzbarth I, Bock P, Haist V, Stein VM, Tipold A, Wewetzer K, Baumgartner W, Beineke A (2011) Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury. J Neuropathol Exp Neurol 70(8):703–714. doi:10.1097/NEN.0b013e3182270f8e
Li X, Lin WJ, Chen CY, Si Y, Zhang X, Lu L, Suswam E, Zheng L, King PH (2012) KSRP: a checkpoint for inflammatory cytokine production in astrocytes. Glia 60(11):1773–1784. doi:10.1002/glia.22396
Klusman I, Schwab ME (1997) Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res 762(1–2):173–184
Busch SA, Silver J (2007) The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17(1):120–127. doi:10.1016/j.conb.2006.09.004
Baptiste DC, Fehlings MG (2006) Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23(3–4):318–334. doi:10.1089/neu.2006.23.318
Hurlbert RJ (2001) The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 26(24 Suppl):S39–S46
Hawryluk GW, Rowland J, Kwon BK, Fehlings MG (2008) Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus 25(5):E14. doi:10.3171/foc.2008.25.11.e14
Tator CH (2006) Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery 59 (5):957–982 (discussion 982–957). doi:10.1227/01.neu.0000245591.16087.89
Lammertse DP (2004) Update on pharmaceutical trials in acute spinal cord injury. J Spinal Cord Med 27(4):319–325
Karimi-Abdolrezaee S, Billakanti R (2012) Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol 46(2):251–264. doi:10.1007/s12035-012-8287-4
Iordache F, Buzila C, Constantinescu A, Andrei E, Maniu H (2012) Histone deacetylase (HDAC) inhibitors down-regulate endothelial lineage commitment of umbilical cord blood derived endothelial progenitor cells. Int J Mol Sci 13(11):15074–15085. doi:10.3390/ijms131115074
White RE, Jakeman LB (2008) Don’t fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci 26(2–3):197–214
Renault-Mihara F, Okada S, Shibata S, Nakamura M, Toyama Y, Okano H (2008) Spinal cord injury: emerging beneficial role of reactive astrocytes’ migration. Int J Biochem Cell Biol 40(9):1649–1653. doi:10.1016/j.biocel.2008.03.009
Davis SJ, Khangure MS (1994) A review of magnetic resonance imaging in spinal trauma. Australas Radiol 38(4):241–253
Radojicic M, Nistor G, Keirstead HS (2007) Ascending central canal dilation and progressive ependymal disruption in a contusion model of rodent chronic spinal cord injury. BMC Neurol 7:30. doi:10.1186/1471-2377-7-30
Ohta K, Fujimura Y, Nakamura M, Watanabe M, Yato Y (1999) Experimental study on MRI evaluation of the course of cervical spinal cord injury. Spinal Cord 37(8):580–584
Arnold SA, Hagg T (2011) Anti-inflammatory treatments during the chronic phase of spinal cord injury improve locomotor function in adult mice. J Neurotrauma 28(9):1995–2002. doi:10.1089/neu.2011.1888
Suda S, Katsura K, Kanamaru T, Saito M, Katayama Y (2013) Valproic acid attenuates ischemia-reperfusion injury in the rat brain through inhibition of oxidative stress and inflammation. Eur J Pharmacol 707(1–3):26–31. doi:10.1016/j.ejphar.2013.03.020
Ververis K, Hiong A, Karagiannis TC, Licciardi PV (2013) Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics 7:47–60. doi:10.2147/btt.s29965
David S, Lopez-Vales R, Wee Yong V (2012) Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol 109:485–502. doi:10.1016/b978-0-444-52137-8.00030-9
David S, Zarruk JG, Ghasemlou N (2012) Inflammatory pathways in spinal cord injury. Int Rev Neurobiol 106:127–152. doi:10.1016/b978-0-12-407178-0.00006-5
Czlonkowska A, Kurkowska-Jastrzebska I (2011) Inflammation and gliosis in neurological diseases—clinical implications. J Neuroimmunol 231(1–2):78–85. doi:10.1016/j.jneuroim.2010.09.020
Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang W, Lu RB, Hong JS (2008) Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol 11(8):1123–1134. doi:10.1017/s1461145708009024
Abdanipour A, Schluesener HJ, Tiraihi T (2012) Effects of valproic acid, a histone deacetylase inhibitor, on improvement of locomotor function in rat spinal cord injury based on epigenetic science. Iran Biomed J 16(2):90–100
Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY (2012) Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121(5):818–829. doi:10.1111/j.1471-4159.2012.07731.x
Khalatbary AR, Tiraihi T (2007) Localization of bone marrow stromal cells in injured spinal cord treated by intravenous route depends on the hemorrhagic lesions in traumatized spinal tissues. Neurol Res 29(1):21–26. doi:10.1179/016164107x165642
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Bose P, Parmer R, Thompson FJ (2002) Velocity-dependent ankle torque in rats after contusion injury of the midthoracic spinal cord: time course. J Neurotrauma 19(10):1231–1249. doi:10.1089/08977150260338029
Liedtke W, Edelmann W, Chiu FC, Kucherlapati R, Raine CS (1998) Experimental autoimmune encephalomyelitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. Am J Pathol 152(1):251–259
Hansmann ML, Wacker HH, Gralla J, Lumbeck H, Kossmahl M, Heidebrecht HJ, Radzun HJ, Parwaresch MR (1991) Ki-B5: a monoclonal antibody unrelated to CD45 recognizes normal and neoplastic human B cells in routine paraffin sections. Blood 77(4):809–817
Aherne WA, Dunnill MS (1982) Morphometry. Edward Arnold, London
Uchihara T (2007) Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol 113(5):483–499. doi:10.1007/s00401-007-0200-2
Naghdi M, Tiraihi T, Namin SA, Arabkheradmand J (2009) Transdifferentiation of bone marrow stromal cells into cholinergic neuronal phenotype: a potential source for cell therapy in spinal cord injury. Cytotherapy 11(2):137–152. doi:10.1080/14653240802716582
Lee MS, Lee YJ, Kim BJ, Shin KJ, Chung BC, Baek DJ, Jung BH (2009) The relationship between glucuronide conjugate levels and hepatotoxicity after oral administration of valproic acid. Arch Pharm Res 32(7):1029–1035. doi:10.1007/s12272-009-1708-x
Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, Moore AN (2010) Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 5(6):e11383. doi:10.1371/journal.pone.0011383
Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q (2011) Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res 1396:60–68. doi:10.1016/j.brainres.2011.03.040
Yu SH, Cho DC, Kim KT, Nam KH, Cho HJ, Sung JK (2012) The neuroprotective effect of treatment of valproic Acid in acute spinal cord injury. J Korean Neurosurg Soc 51(4):191–198. doi:10.3340/jkns.2012.51.4.191
van Bergeijk J, Haastert K, Grothe C, Claus P (2006) Valproic acid promotes neurite outgrowth in PC12 cells independent from regulation of the survival of motoneuron protein. Chem Biol Drug Des 67(3):244–247. doi:10.1111/j.1747-0285.2006.00369.x
Su Z, Niu W, Liu ML, Zou Y, Zhang CL (2014) In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 5:3338. doi:10.1038/ncomms4338
Wang C, Luan Z, Yang Y, Wang Z, Cui Y, Gu G (2011) Valproic acid induces apoptosis in differentiating hippocampal neurons by the release of tumor necrosis factor-alpha from activated astrocytes. Neurosci Lett 497(2):122–127. doi:10.1016/j.neulet.2011.04.044
Thuret S, Moon LD, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7(8):628–643. doi:10.1038/nrn1955
Okonkwo DO, Reece TB, Laurent JJ, Hawkins AS, Ellman PI, Linden J, Kron IL, Tribble CG, Stone JR, Kern JA (2006) A comparison of adenosine A2A agonism and methylprednisolone in attenuating neuronal damage and improving functional outcome after experimental traumatic spinal cord injury in rabbits. J Neurosurg Spine 4(1):64–70. doi:10.3171/spi.2006.4.1.64
Oudega M, Vargas CG, Weber AB, Kleitman N, Bunge MB (1999) Long-term effects of methylprednisolone following transection of adult rat spinal cord. Eur J Neurosci 11(7):2453–2464
Wilson JR, Forgione N, Fehlings MG (2013) Emerging therapies for acute traumatic spinal cord injury. CMAJ 185(6):485–492. doi:10.1503/cmaj.121206
Constantini S, Young W (1994) The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg 80(1):97–111. doi:10.3171/jns.1994.80.1.0097
Kaynar MY, Erdincler P, Tadayyon E, Belce A, Gumustas K, Ciplak N (1998) Effect of nimodipine and N-acetylcysteine on lipid peroxidation after experimental spinal cord injury. Neurosurg Rev 21(4):260–264
Massagli TL, Cardenas DD (1999) Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil 80(9):998–1000
Lu DC, Yuan XH, Li HJ, Wei XL (2005) An experimental study of the neuroprotective effect of FK506 on acute spinal cord injury in dogs. Zhonghua Wai Ke Za Zhi 43(16):1088–1090
Springer JE, Rao RR, Lim HR, Cho SI, Moon GJ, Lee HY, Park EJ, Noh JS, Gwag BJ (2010) The functional and neuroprotective actions of Neu 2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. J Neurotrauma 27(1):139–149. doi:10.1089/neu.2009.0952
Esquivel-Aguilar A, Castaneda-Hernandez G, Martinez-Cruz A, Franco-Bourland RE, Madrazo I, Guizar-Sahagun G (2011) Early administration of l-arginine in experimental acute spinal cord injury impairs long-term motor function recovery. J Trauma 70(5):1198–1202. doi:10.1097/TA.0b013e3181e3e5c1
Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L (2011) A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28(5):787–796. doi:10.1089/neu.2011.1765
Yoon DH, Kim YS, Young W (1999) Therapeutic time window for methylprednisolone in spinal cord injured rat. Yonsei Med J 40(4):313–320
Gorio A, Madaschi L, Di Stefano B, Carelli S, Di Giulio AM, De Biasi S, Coleman T, Cerami A, Brines M (2005) Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci USA 102(45):16379–16384. doi:10.1073/pnas.0508479102
Abdul Roda M, Sadik M, Gaggar A, Hardison MT, Jablonsky MJ, Braber S, Blalock JE, Redegeld FA, Folkerts G, Jackson PL (2014) Targeting prolyl endopeptidase with valproic acid as a potential modulator of neutrophilic inflammation. PLoS One 9(5):e97594. doi:10.1371/journal.pone.0097594
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264
Hall ED, Wolf DL, Braughler JM (1984) Effects of a single large dose of methylprednisolone sodium succinate on experimental posttraumatic spinal cord ischemia. Dose–response and time–action analysis. J Neurosurg 61(1):124–130. doi:10.3171/jns.1984.61.1.0124
Kaptanoglu E, Caner H, Solaroglu I, Kilinc K (2005) Mexiletine treatment-induced inhibition of caspase-3 activation and improvement of behavioral recovery after spinal cord injury. J Neurosurg Spine 3(1):53–56. doi:10.3171/spi.2005.3.1.0053
Cayli SR, Kocak A, Yilmaz U, Tekiner A, Erbil M, Ozturk C, Batcioglu K, Yologlu S (2004) Effect of combined treatment with melatonin and methylprednisolone on neurological recovery after experimental spinal cord injury. Eur Spine J 13(8):724–732. doi:10.1007/s00586-003-0550-y
Siegal T, Siegal T, Lossos F (1990) Experimental neoplastic spinal cord compression: effect of anti-inflammatory agents and glutamate receptor antagonists on vascular permeability. Neurosurgery 26(6):967–970
Lee JK, Johnson CS, Wrathall JR (2007) Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol 203(2):502–511. doi:10.1016/j.expneurol.2006.09.003
Cote MP, Detloff MR, Wade RE Jr, Lemay MA, Houle JD (2012) Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol 3:330. doi:10.3389/fphys.2012.00330
Weishaupt N, Blesch A, Fouad K (2012) BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol 238(2):254–264. doi:10.1016/j.expneurol.2012.09.001
Chen XY, Feng-Chen KC, Chen L, Stark DM, Wolpaw JR (2001) Short-Term and medium-term effects of spinal cord tract transections on soleus H-reflex in freely moving rats. J Neurotrauma 18(3):313–327. doi:10.1089/08977150151070973
Hu Y, Wen CY, Li TH, Cheung MM, Wu EX, Luk KD (2011) Somatosensory-evoked potentials as an indicator for the extent of ultrastructural damage of the spinal cord after chronic compressive injuries in a rat model. Clin Neurophysiol 122(7):1440–1447. doi:10.1016/j.clinph.2010.12.051
Mothe AJ, Tator CH (2005) Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 131(1):177–187. doi:10.1016/j.neuroscience.2004.10.011
Takahashi M, Arai Y, Kurosawa H, Sueyoshi N, Shirai S (2003) Ependymal cell reactions in spinal cord segments after compression injury in adult rat. J Neuropathol Exp Neurol 62(2):185–194
Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J (2008) Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6(7):e182. doi:10.1371/journal.pbio.0060182
Dervan AG, Roberts BL (2003) Reaction of spinal cord central canal cells to cord transection and their contribution to cord regeneration. J Comp Neurol 458(3):293–306. doi:10.1002/cne.10594
Attar A, Kaptanoglu E, Aydin Z, Ayten M, Sargon MF (2005) Electron microscopic study of the progeny of ependymal stem cells in the normal and injured spinal cord. Surg Neurol 64(Suppl 2):S28–S32. doi:10.1016/j.surneu.2005.07.057
Lu WH, Wang CY, Chen PS, Wang JW, Chuang DM, Yang CS, Tzeng SF (2013) Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia. J Neurosci Res 91(5):694–705. doi:10.1002/jnr.23200
Fatemi SH, Folsom TD, Reutiman TJ, Pandian T, Braun NN, Haug K (2008) Chronic psychotropic drug treatment causes differential expression of connexin 43 and GFAP in frontal cortex of rats. Schizophr Res 104(1–3):127–134. doi:10.1016/j.schres.2008.05.016
Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M (2008) Loss of astrocytic domain organization in the epileptic brain. J Neurosci 28(13):3264–3276. doi:10.1523/jneurosci.4980-07.2008
Faulkner J, Keirstead HS (2005) Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol 15(2):131–142. doi:10.1016/j.trim.2005.09.007
Ximenes JC, de Oliveira Goncalves D, Siqueira RM, Neves KR, Santos Cerqueira G, Correia AO, Felix FH, Leal LK, de Castro Brito GA, da Graca Naffah-Mazzacorati M, Viana GS (2013) Valproic acid: an anticonvulsant drug with potent antinociceptive and anti-inflammatory properties. Naunyn Schmiedebergs Arch Pharmacol 386(7):575–587. doi:10.1007/s00210-013-0853-4
Zhang Z, Zhang ZY, Wu Y, Schluesener HJ (2012) Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience 221:140–150. doi:10.1016/j.neuroscience.2012.07.013
Pannu R, Barbosa E, Singh AK, Singh I (2005) Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res 79(3):340–350. doi:10.1002/jnr.20345
Pineau I, Lacroix S (2007) Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500(2):267–285. doi:10.1002/cne.21149
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155. doi:10.1523/jneurosci.3547-03.2004
Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M (2013) Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38(3):555–569. doi:10.1016/j.immuni.2013.02.012
Medzhitov R, Horng T (2009) Transcriptional control of the inflammatory response. Nat Rev Immunol 9(10):692–703. doi:10.1038/nri2634
Wu C, Li A, Leng Y, Li Y, Kang J (2012) Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA Cell Biol 31(4):592–599. doi:10.1089/dna.2011.1401
Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A (2013) The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma 30(15):1311–1324. doi:10.1089/neu.2012.2651
Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, Saunders NR (2012) Pathological changes in the white matter after spinal contusion injury in the rat. PLoS One 7(8):e43484. doi:10.1371/journal.pone.0043484
Penas C, Verdu E, Asensio-Pinilla E, Guzman-Lenis MS, Herrando-Grabulosa M, Navarro X, Casas C (2011) Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience 178:33–44. doi:10.1016/j.neuroscience.2011.01.012
Vanzani MC, Iacono RF, Caccuri RL, Troncoso AR, Berria MI (2006) Regional differences in astrocyte activation in HIV-associated dementia. Medicina (B Aires) 66(2):108–112
Acknowledgments
The project was funded by Shefa Neurosciences Research Center at Khatam Al-Anbia Hospital, Tehran, Iran (Grant # 86-N-105). We are also grateful for the support of the Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. We would like express our deep gratitude for Mrs. HH AliAkbar for editing the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darvishi, M., Tiraihi, T., Mesbah-Namin, S.A. et al. Decreased GFAP Expression and Improved Functional Recovery in Contused Spinal Cord of Rats Following Valproic Acid Therapy. Neurochem Res 39, 2319–2333 (2014). https://doi.org/10.1007/s11064-014-1429-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1429-5