Abstract
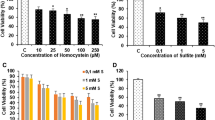
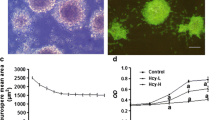
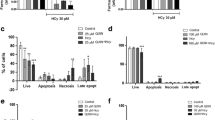
Epidemiological and experimental evidence indicated that hyperhomocysteinemia is associated with neurodegeneration. However, homocysteine neurotoxic effects have been so far investigated mostly by employing homocysteine concentrations (≥100 µM) much higher than homocysteine mean plasma levels (20 µM) observed in patients with neurodegenerative disorders. While evaluating the effects of a prolonged exposure to ~20 µM homocysteine in neuronal-like differentiated SH-SY5Y cells, we observed a 35 % loss of cell viability and a four-fold increase in reactive oxygen species levels in cells incubated with homocysteine for five days compared with controls. Moreover, homocysteine increased by 30 % and around two-fold, respectively, the Comet-positive cell number and DNA damage indexes (tail length, T-DNA, olive tail moment) compared with controls. Cell response to homocysteine-induced DNA damage involved the up-regulation of Bax and, at a greater extent, Bcl-2, but not caspase-3, in association with a p53-independent increase of p21 levels; concomitantly, also p16 levels were increased. When looking at time-dependent changes in cyclin expression, we found that a significant up-regulation of cyclins D1, A1, E1, but not B1, concomitant with p21 down-regulation, occurred in cells incubated with homocysteine for three days. However, in line with the observed increase of p21 and p16 levels, a five days incubation with homocysteine induced cyclin down-regulation accompanied by a strong reduction of phosphorylated pRB amounts. These results suggest that, when prolonged, the exposure of neuronal-like cells to mildly elevated homocysteine concentrations triggers oxidative and genotoxic stress involving an early induction of cyclins, that is late repressed by G1-S check-point regulators.






Similar content being viewed by others
References
Selhub J (2008) Public health significance of elevated homocysteine. Food Nutr Bull 29(2 Suppl):S116–S125
Ientile R, Curro’ M, Ferlazzo N, Condello S, Caccamo D, Pisani F (2010) Homocysteine, vitamin determinants and neurological diseases. Front Biosci (Schol Ed) 2:359–372
Wald DS, Kasturiratne A, Simmonds M (2011) Serum homocysteine and dementia:meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement 7(4):412–417
Zhuo JM, Wang H, Praticò D (2011) Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci 32(9):562–571
Mattson MP (2006) Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal 8(11–12):1997–2006
Boldyrev AA (2009) Molecular mechanisms of homocysteine toxicity. Biochemistry (Mosc) 74(6):589–598
Sharma P, Senthilkumar RD, Brahmachari V, Sundaramoorthy E, Mahajan A, Sharma A, Sengupta S (2006) Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis 5:1–19
Rabaneda LG, Carrasco M, López-Toledano MA, Murillo-Carretero M, Ruiz FA, Estrada C, Castro C (2008) Homocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expression. FASEB J 22(11):3823–3835
Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC (2006) Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol 290(3):H1206–H1213
Beard RS Jr, Reynolds JJ, Bearden SE (2011) Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 118(7):2007–2014
Condello S, Currò M, Ferlazzo N, Caccamo D, Satriano J, Ientile R (2011) Agmatine effects on mitochondrial membrane potential and NF-κB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. J Neurochem 116(1):67–75
Picerno I, Chirico C, Condello S, Visalli G, Ferlazzo N, Gorgone G, Caccamo D, Ientile R (2007) Homocysteine induces DNA damage and alterations in proliferative capacity of T-lymphocytes: a model for immunosenescence? Biogerontology 8(2):111–119
Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M (2001) Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci 24:25–31
Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20(18):6920–6926
Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP (2002) Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci 22(5):1752–1762
Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP, Krieglstein J (2003) Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci 23(24):8586–8595
Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R Jr, Gorospe M, Mattson MP (2004) Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41(4):549–561
Zieminska E, Lazarewicz JW (2006) Excitotoxic neuronal injury in chronic homocysteine neurotoxicity studied in vitro: the role of NMDA and group I metabotropic glutamate receptors. Acta Neurobiol Exp (Wars) 66(4):301–309
Zieminska E, Matyja E, Kozlowska H, Stafiej A, Lazarewicz JW (2006) Excitotoxic neuronal injury in acute homocysteine neurotoxicity: role of calcium and mitochondrial alterations. Neurochem Int 48(6–7):491–497
Slomka M, Zieminska E, Lazarewicz J (2008) Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 68(1):1–9
Kuszczyk M, Gordon-Krajcer W, Lazarewicz JW (2009) Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem Int 55(1–3):174–180
Ye W, Blain SW (2010) S phase entry causes homocysteine-induced death while ataxia telangiectasia and Rad3 related protein functions anti-apoptotically to protect neurons. Brain 133(Pt 8):2295–2312
Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8:368–378
Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, Tulchinsky E, Jones GD, Roninson IB, Macip S (2012) Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem 287(13):9845–9854
Yang X, Wang W, Qin JJ, Wang MH, Sharma H, Buolamwini JK, Wang H, Zhang R (2012) JKA97, a novel benzylidene analog of harmine, exerts anti-cancer effects by inducing G1 arrest, apoptosis, and p53-independent up-regulation of p21. PLoS One 7(4):e34303
Russo A, Esposito D, Catillo M, Pietropaolo C, Crescenzi E, Russo G (2013) Human rpL3 induces G1/S arrest or apoptosis by modulating p21 (waf1/cip1) levels in a p53-independent manner. Cell Cycle 12(1):76–87
Condello S, Currò M, Ferlazzo N, Costa G, Visalli G, Caccamo D, Pisani LR, Costa C, Calabresi P, Ientile R, Pisani F (2013) Protective effects of Zonisamide against Rotenone-induced neurotoxicity. Neurochem Res 38(12):2631–2639
Acknowledgments
We thank Prof. Isa Picerno from University of Messina for generously providing us Cyclin E and Cyclin A1 antibodies.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Currò and A. Gugliandolo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Currò, M., Gugliandolo, A., Gangemi, C. et al. Toxic Effects of Mildly Elevated Homocysteine Concentrations in Neuronal-Like Cells. Neurochem Res 39, 1485–1495 (2014). https://doi.org/10.1007/s11064-014-1338-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1338-7