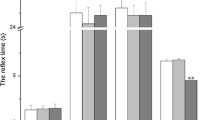
Abstract
Pharmacological treatment is a therapeutic approach to improving nerve regeneration and functional recovery after peripheral nerve crush injury. The objective of the present study was to investigate the effects of the polypeptides isolated from Achyranthes bidentata Blume (abbreviated as ABPP) on rat sciatic crush injury and to test the possible involvement of neurotrophic factors. After surgical crush injury, rats received daily intraperitoneal injection of 0.2 ml saline containing 2 mg ABPP, 1 μg nerve growth factor (NGF) or no additive. The results from walking track analysis, electrophysiological assessment and histological evaluation indicated that the repair outcomes by ABPP treatment were close to those by NGF treatment, but better than those by treatment with saline alone. The quantitative real-time RT-PCR was used to monitor the mRNA expression of growth associated protein in the crush nerves and the mRNA expression of NGF, brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), tyrosine kinase (Trk)A and TrkB in the dorsal root ganglia (DRGs) at L4–L6. The mRNA expression of these genes in the crush nerve sample and DRGs sample was higher after treatment with ABPP or NGF than after treatment with saline alone. Our findings suggest that ABPP might protect peripheral nerve against crush injury through stimulating release of neurotrophic factors and the other cytokines.





Similar content being viewed by others
References
Gu XS, Ding F, Yang YM, Liu J (2011) Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol 93:204–230
Jacob JM, Zhou Q, Liu Y (2000) Novel method for the labeling of distant neuromuscular junctions. J Neurosci Res 61(1):61–66
Xie Y, Yeo TT, Zhang C, Yang T, Tisi MA, Massa SM, Longo FM (2001) The leukocyte common antigen-related protein tyrosine phosphotase receptor regulates regenerative neurite outgrowth in vivo. J Neurosci 21(14):5130–5138
Gordon T (2009) The role of neurotrophic factors in nerve regeneration. Neurosurg Focus 26(2):1–10
Fu SY, Gordon T (1997) The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 14(1–2):67–116
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194(Pt1):1–14
Höke A, Gordon T, Zochodne DW, Sulaiman OAR (2002) A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol 173:77–85
Wei SY, Xf Yin, Kou YH, Jiang BG (2009) Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. J Ethnopharmacol 123:51–54
Lu MC, Yao CH, Wang SH, Lai YL, Tsai CC, Chen YS (2010) Effect of Astragalus membranaceous in rats o peripheral nerve regeneration: in vitro and in vivo studies. J Trauma 68:434–440
Committee of National Pharmacopoeia (2005) Pharmacopoeia of the People’s Republic of China, Chemical Industry press, Beijing, p 49
Li J, Qi H, Qi LW, Yi L, Li P (2007) Simultaneous determination of main phytoecdysones and triterpenoids in radix achyranthis bidentatae by high-performance liquid chromatography with diode array-evaporative light scattering detectors and mass spectrometry. Anal Chim Acta 596(2):264–272
Shen H, Yuan Y, Ding F, Liu J, Gu X (2008) The protective effects of Achyranthes bidentata polypeptides against NMDA-induced cell apoptosis in cultured hippocampal neurons through differential modulation of NR2A- and NR2B-containing NMDA receptors. Brain Res Bull 77(5):274–281
Yuan Y, Shen H, Yao J, Hu N, Ding F, Gu X (2010) The protective effects of Achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Res Bull 81(1):25–32
Chen ZW, Wang MS (1995) Effects of nerve growth factor on crushed sciatic nerve regeneration in rats. Microsurgery 16(8):547–551
Yin ZS, Zhang H, Bo W, Gao W (2010) Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. AJNR Am J Neuroradiol 31(3):509–515
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83(1):129–138
Callizot N, Andriambeloson E, Glass J, Revel M, Ferro P, Cirillo R, Vitte PA, Dreano M (2008) Interleukin-6 protects against paclitaxel, cisplatin and vincristine-induced neuropathies without impairing chemotherapeutic activity. Cancer Chemother Pharmacol 62(6):995–1007
Baptista AF, Gomes JRdS, Oliveira JT, Santos SMG, Vannier-Santos MA, Martinez AMB (2007) A new approach to assess function after sciatic nerve lesion in the mouse-daptation of the sciatic static index. J Neurosci Methods 161(2):259–264
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods 25(4):402–408
Rodrigues Hell RC, Silva Costa MM, Goes AM, Oliveira ALR (2009) Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol Dis 33(2):290–300
Allodi I, Udina E, Navarro X (2012) Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol 98(1):16–37
Miao G, Mace J, Kirby M, Hopper A, Peverini R, Chinnock R, Shapiro J, Hathout E (2005) Beneficial effects of nerve growth factor on islet transplantation. Transpl Proc 37(8):3490–3492
Tuszynski MH, HS U, Amaral DG, Gage FH (1990) Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci 10(11):3604–3614
Oudega M, Hagg T (1996) Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol 140(2):218–229
Connor B, Dragunow M (1998) The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev 27(1):1–39
Taglialatela G, Foreman PJ, Perez-Polo JR (1997) Effect of a long-term nerve growth factor treatment on body weight, blood pressure, and serum corticosterone in rats. Int J Dev Neurosci 15(6):703–710
Sun W, Sun C, Zhao H, Lin H, Han Q, Wang J, Ma H, Chen B, Xiao Z, Dai J (2009) Improvement of sciatic nerve regeneration using laminin-binding human NGF-beta. PLoS One 4(7):e6180
Martins RS, Siqueira MG, da Silva CF, Plese JP (2006) Correlation between parameters of electrophysiological, histomorphometric and sciatic functional index evaluations after rat sciatic nerve repair. Arq Neuropsiquiatr 64(3B):750–756
Irwin N, Chao S, Goritchenko L, Horiuchi A, Greengard P, Nairn AC, Benowitz LI (2002) Nerve growth factor controls GAP-43 mRNA stability via the phosphoprotein ARPP-19. Proc Natl Acad Sci USA 99(19):12427–12431
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20(2):84–91
Yuan Y, Zhou SL, Gu XS, Ding F (2009) Polypeptides of Achyranthes bidentata Blume promotes the neurite growth of rat hippocampal neurons. Acta Anatomica Sinica 40(5):696–700
Wright DE, Snider WD (1995) Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351(3):329–338
Conover JC, Yancopoulos GD (1997) Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev Neurosci 8(1):13–27
Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, Barrett GL, Kilpatrick TJ, Murray SS (2009) BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci 29(13):4016–4022
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Kashiba H, Senba E (1999) Up- and down-regulation of BDNF mRNA in distinct subgroups of rat sensory neurons after axotomy. NeuroReport 10(17):3561–3565
Kobayashi NR, Bedard AM, Hincke MT, Tetzlaff W (1996) Increased expression of BDNF and trkB mRNA in rat facial motoneurons after axotomy. Eur J Neurosci 8(5):1018–1029
Gu ZZ, Pan YC, Cui JK, Klebuc MJ, Shenaq S, Liu PK (1997) Gene expression and apoptosis in the spinal cord neurons after sciatic nerve injury. Neurochem Int 30(4–5):417–426
Shen H, Chung JM, Coggeshaoll RE, Chung K (1999) Changes in trkA expression in the dorsal root ganglion after peripheral nerve injury. Exp Brain Res 127(2):141–146
Acknowledgments
The financial supports of National Natural Science Foundation of China (Grant No. 30970996), “Liu Da Ren Cai Gao Feng” of Jiangsu province (Grant No. Human Resources and Social Security Department in Jiangsu Province (2010) No. 555), and “333 Ren Cai Gong Cheng” of Jiangsu province (2011, III-2240) “226 Ren Cai Gong Cheng” of Nantong are gratefully acknowledged. We thank Professor Jie Liu for assistant in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yaxian Wang and Weixing Shen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Shen, W., Yang, L. et al. The Protective Effects of Achyranthes bidentata Polypeptides on Rat Sciatic Nerve Crush Injury Causes Modulation of Neurotrophic Factors. Neurochem Res 38, 538–546 (2013). https://doi.org/10.1007/s11064-012-0946-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0946-3