Abstract
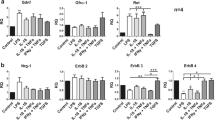
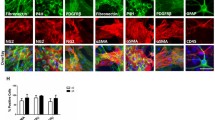
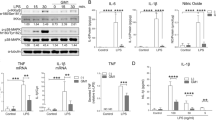
Gangliosides have long been implicated in multiple pathologies affecting the central nervous system. Empirical studies have suggested the possibility that gangliosides, particularly GD3, work in tandem with pro-inflammatory cytokines, especially tumor necrosis factor alpha (TNFα), to initiate or facilitate cell death in the CNS. As a step toward unraveling the metabolic pathways activated in the pathogenesis of brain cell death, we have surveyed gene expression for a host of cytokines and chemokines in primary brain cell cultures exposed to GD3, GD1b, and TNFα for 24 h. An initial screen of 98 genes on a focused mini-array revealed the expression of at least 28 genes related to cell growth, death, or inflammation in our system of mixed cells cultured from neonatal rat brains. Clear evidence of a differential response to the gangliosides or TNFα was seen in 12 genes. Quantitative PCR was used to validate the response of six of these genes. We found that both GD3 and GD1b, but not TNFα, up-regulated expression of macrophage inflammatory protein 3 (MIP3A) and interleukin-1 receptor 1 (IL1R1), but down-regulated fibroblast growth factor 13 (FGF13). The expression of FGF receptor activating protein 1 (FRAG1) and interleukin-3 receptor alpha (IL3RA) was down-regulated by GD3. Exposure to TNFα resulted in a dramatic up-regulation of IL3RA and chemokine ligand 2 (CCL2), both of which have been implicated in multiple sclerosis. Our results provide strong evidence that the expression of these genes might be critical links in the metabolic cascades leading to cell degeneration and death in the brain.





Similar content being viewed by others
Abbreviations
- CCL2:
-
Chemokine ligand 2
- CD123:
-
Cluster of differentiation 123
- COX-2:
-
Cyclooxygenase 2
- FGF2 (bFGF):
-
Fibroblast growth factor 2
- FGF13:
-
Fibroblast growth factor 13
- FRAG1:
-
Fibroblast growth factor receptor activating protein 1
- GM1:
-
Galβ3GalNAcβ4(Neu5Acα3)Galβ4GlcCer
- GD3:
-
Neu5Acα8Neu5Acα3Galβ4GlcCer
- GD1a:
-
Neu5Acα3Galβ3GalNAcβ4(Neu5Acα3)Galβ4GlcCer
- GD1b:
-
Galβ3GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4GlcCer
- GT1b:
-
Neu5Acα3Galβ3GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4GlcCer
- IFN-β:
-
Interferon beta
- IFN-γ:
-
Interferon gamma
- IL1R1:
-
Interleukin 1 receptor 1
- IL3RΑ:
-
Interleukin 3 receptor alpha
- MAG4:
-
Myelin-associated glycoprotein 4
- MIP3A:
-
Macrophage inflammatory protein 3a
- MTS:
-
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- NO:
-
Nitric Oxide
- qPCR:
-
Quantitative (real time) polymerase chain reaction
- TNFα:
-
Tumor necrosis factor alpha
- TNFR2:
-
Tumor necrosis factor receptor 2
References
Holmgren J, Lonnroth I, Svennerholm L (1973) Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis 5:77–78
Holmgren J, Lonnroth I, Svennerholm L (1973) Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun 8:208–214
Bukrinskaia AG, Kornilaeva GV, Vorkunova NK et al (1982) Gangliosides–specific receptors for the influenza virus. Vopr Virusol 27:661–666
Poulain B (1994) Molecular mechanism of action of tetanus toxin and botulinum neurotoxins. Pathol Biol (Paris) 42:173–182
Hadjiconstantinou M, Neff NH (1998) GM1 ganglioside: in vivo and in vitro trophic actions on central neurotransmitter systems. J Neurochem 70:1335–1345
Avrova NF, Victorov IV, Tyurin VA et al (1998) Inhibition of glutamate-induced intensification of free radical reactions by gangliosides: possible role in their protective effect in rat cerebellar granule cells and brain synaptosomes. Neurochem Res 23:945–952
Zaprianova E, Majtenyi K, Deleva D et al (2004) Serum IgG and IgM ganglioside GM1 antibodies in patients with multiple sclerosis. Ideggyogy Sz 57:94–99
Marconi S, Acler M, Lovato L et al (2006) Anti-GD2-like IgM autoreactivity in multiple sclerosis patients. Multiple Scler 12:302–308
Kristal BS, Brown AM (1999) Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J Biol Chem 274:23169–23175
Garcia-Ruiz C, Colell A, Paris R et al (2000) Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. Faseb J 14:847–858
Garcia-Ruiz C, Colell A, Morales A et al (2002) Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J Biol Chem 277:36443–36448
Colell A, Garcia-Ruiz C, Roman J et al (2001) Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. Faseb J 15:1068–1070
Malisan F, Testi R (2002) GD3 ganglioside and apoptosis. Biochim Biophys Acta 1585:179–187
Melchiorri D, Martini F, Lococo E et al (2002) An early increase in the disialoganglioside GD3 contributes to the development of neuronal apoptosis in culture. Cell Death Differ 9:609–615
Simon BM, Malisan F, Testi R (2002) Disialoganglioside GD3 is released by microglia and induces oligodendrocyte apoptosis. Cell Death Differ 9:758–767
Hasegawa T, Sugeno N, Takeda A et al (2007) Role of Neu4L sialidase and its substrate ganglioside GD3 in neuronal apoptosis induced by catechol metabolites. FEBS Lett 581:406–412
Seyfried TN, Yu RK (1985) Ganglioside GD3: structure, cellular distribution, and possible function. Mol Cell Biochem 68:3–10
Morales A, Colell A, Mari M et al (2004) Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj J 20:579–588
Kim OS, Park EJ, Joe EH et al (2002) JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 277:40594–40601
Min KJ, Pyo HK, Yang MS et al (2004) Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia 48:197–206
Pyo H, Joe E, Jung S et al (1999) Gangliosides activate cultured rat brain microglia. J Biol Chem 274:34584–34589
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65:2702–2720
Rasband WS (1997-2011) ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA
Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW (1998) Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci 18:5804–5816
Terao Y, Ohta H, Oda A et al (2009) Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res 64:75–82
Ambrosini E, Remoli ME, Giacomini E et al (2005) Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 64:706–715
Raivich G, Bohatchek M, Werner A et al (2003) Lymphocyte infiltration in the injured brain: role of proinflammatory cytokines. J Neurosci Res 72:726–733
Muzio M, Polentarutti N, Bosisio D et al (2000) Toll-like receptor family and signalling pathway. Biochem Soc Trans 28:563–566
Nyati KK, Prasad KN, Verma A et al (2009) Association of TLR4 Asp299Gly and Thr399Ile polymorphisms with Guillain-Barre syndrome in Northern Indian population. J Neuroimmunol 218:116–119
O’Neill L (2000) The toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans 28:557–563
O’Neill LA (2002) Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol 270:47–61
Rusnati M, Tanghetti E, Urbinati C et al (1999) Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides: biochemical characterization and biological consequences in endothelial cell cultures. Mol Biol Cell 10:313–327
Gecz J, Baker E, Donnelly A et al (1999) Fibroblast growth factor homologous factor 2 (FHF2): gene structure, expression and mapping to the Borjeson-Forssman-Lehmann syndrome region in Xq26 delineated by a duplication breakpoint in a BFLS-like patient. Hum Genet 104:56–63
Rush AM, Wittmack EK, Tyrrell L et al (2006) Differential modulation of sodium channel Na(v)1.6 by two members of the fibroblast growth factor homologous factor 2 subfamily. Eur J Neurosci 23:2551–2562
Goetz R, Dover K, Laezza F et al (2009) Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J Biol Chem 284:17883–17896
Ishii H, Inageta T, Mimori K et al (2005) Frag1, a homolog of alternative replication factor C subunits, links replication stress surveillance with apoptosis. Proc Natl Acad Sci USA 102:9655–9660
Huang YM, Adikari S, Bave U et al (2005) Multiple sclerosis: interferon-beta induces CD123(+)BDCA2- dendritic cells that produce IL-6 and IL-10 and have no enhanced type I interferon production. J Neuroimmunol 158:204–212
Lopez C, Comabella M, Al-zayat H et al (2006) Altered maturation of circulating dendritic cells in primary progressive MS patients. J Neuroimmunol 175:183–191
Subileau EA, Rezaie P, Davies HA et al (2009) Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol 68:227–240
Dogan RN, Elhofy A, Karpus WJ (2008) Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol 180:7376–7384
Ulrich-Bott B, Wiegandt H (1984) Micellar properties of glycosphingolipids in aqueous media. J Lipid Res 25:1233–1245
Leskawa KC, Erwin RE, Leon A et al (1989) Incorporation of exogenous ganglioside GM1 into neuroblastoma membranes: inhibition by calcium ion and dependence upon membrane protein. Neurochem Res 14:547–554
Saqr HE, Pearl DK, Yates AJ (1993) A review and predictive models of ganglioside uptake by biological membranes. J Neurochem 61:395–411
Young HP, Christian ZF, Cabeza R, Irwin LN (1998) Uptake of exogenous gangliosides by rat brain synaptosomes. Neurochem Res 23:1515–1520
Bhunia AK, Schwarzmann G, Chatterjee S (2002) GD3 recruits reactive oxygen species to induce cell proliferation and apoptosis in human aortic smooth muscle cells. J Biol Chem 277:16396–16402
Schwarzmann G, Hofmann P, Putz U, Albrecht B (1995) Demonstration of direct glycosylation of nondegradable glucosylceramide analogs in cultured cells. J Biol Chem 270:21271–21276
Avrova NF, Sokolova TV, Vlasova YA et al (2010) Protective and antioxidative effects of GM1 ganglioside in PC12 cells exposed to hydrogen peroxide are mediated by Trk tyrosine kinase. Neurochem Res 35:85–98
Acknowledgments
This work made use of facilities supported by a grant from the Research Centers at Minority Institutions program of the National Center for Research Resources (NIH # G12RR008124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Byers, D.M., Gorbet, J.C. & Irwin, L.N. Disialogangliosides and TNFα Alter Gene Expression for Cytokines and Chemokines in Primary Brain Cell Cultures. Neurochem Res 37, 214–222 (2012). https://doi.org/10.1007/s11064-011-0587-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0587-y