Abstract
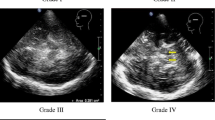
Increased area of the substantia nigra (SN) associated to iron deposition has been proposed as a specific marker for Parkinson’s disease (PD). Echogenicity, assessed by transcranial sonography (TCS), has been used to measure such an iron deposition. On the other hand, ferroxidase activity is known to play a role in brain iron metabolism and thus could be involved in increased SN echogenicity of PD patients. The present study was conducted to search for a possible correlation between both markers: TCS of SN and plasma ferroxidase activity. Twenty-one PD patients and 13 healthy volunteers (HV) were included. Mean SN sonographic areas were 0.31 cm2 for PD patients and 0.12 cm2 for HV (P < 0.001), while plasma ferroxidase activity was reduced in PD patients (P < 0.001). Interestingly, plasma ferroxidase activity was inversely correlated with the SN size by TCS (R2 = 0.31), suggesting a relationship between the two markers.


Similar content being viewed by others
References
Tanner CM, Aston DA (2000) Epidemiology of Parkinson’s disease and akinetic syndromes. Curr Opin Neurol 13:427–430
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Berg D, Siefker C, Becker G (2001) Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 248:684–689
Walter U, Dressler D, Wolters A, Wittstock M, Benecke R (2007) Transcranial brain sonography findings in clinical subgroups of Idiopathic Parkinson’s disease. Mov Disord 22:48–54
Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, Tampellini D, Riederer P, Gerlach M, Becker G (2005) In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord 20:1278–1285
Mukhopadhyay C, Attieh ZK, Fox PL (1998) Role of ceruloplasmin in cellular iron uptake. Science 279:714–717
Tajima K, Kawanami T, Nagai R, Horiuchi S, Kato T (1999) Hereditary ceruloplasmin deficiency increases advanced glycation end products in the brain. Neurology 53:619–622
Boll MC, Sotelo J, Otero E, Alcaraz-Zubeldia M, Rios C (1999) Reduced ferroxidase activity in the cerebrospinal fluid from patients with Parkinson’s disease. Neurosci Lett 265:155–158
Tórsdóttir G, Kristinsson J, Sveinbjörnsdóttir S, Snaedal J, Jóhannesson T (1999) Copper, ceruloplasmin, superoxide dismutase and iron parameters in Parkinson’s disease. Pharmacol Toxicol 85:239–243
Hochstrasser H, Tomiuk J, Walter U, Behnke S, Spiegel J, Krüger R, Becker G, Riess O, Berg D (2005) Functional relevance of ceruloplasmin mutations in Parkinson’s disease. FASEB J 19:1851–1853
Berg D, Behnke S, Walter U (2006) Application of transcranial sonography in extrapyramidal disorders: updated recommendations. Ultraschall Med 27:12–19
Tanabe S, Shioiri T, Murakami K, Imanari T (1984) A new method for assay of ferroxidase activity and its application to human and rabbit sera. Chem Pharm Bull (Tokio) 32:4029–4035
Prestel J, Schweitzer KJ, Hofer A, Gasser T, Berg D (2006) Predictive value of transcranial sonography in the diagnosis of Parkinson’s disease. Mov Disord 21:1763–1765
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60:74–77
Tsai CF, Wu RM, Huang YW, Chen LL, Yip PK, Jeng JS (2007) Transcranial color coded sonography helps differentiation between idiopathic Parkinson’s disease and vascular parkinsonism. J Neurol 254:501–507
Walter U, Wittstock M, Benecke R, Dressler D (2002) Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. J Neural Transm 109:191–196
Walter U, Dressler D, Wolters A, Probst T, Grossmann A, Benecke R (2004) Sonographic discrimination of corticobasal degeneration vs. progressive supranuclear palsy. Neurology 63:504–509
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873
Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G (2001) Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem 79:225–236
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–S38
Rivera-Mancía S, Pérez-Neri I, Ríos C, Tristán-López L, Rivera-Espinosa L, Montes S (2010) The transition metals copper and iron in neurodegenerative diseases. Chem Biol Interact 186:184–199
Jin L, Wang J, Zhao L, Jin H, Fei G, Zhang Y, Zeng M, Zhong C (2011) Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain 134:50–58
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Hernández, R., Montes, S., Higuera-Calleja, J. et al. Plasma Ceruloplasmin Ferroxidase Activity Correlates with the Nigral Sonographic Area in Parkinson’s Disease Patients: A Pilot Study. Neurochem Res 36, 2111–2115 (2011). https://doi.org/10.1007/s11064-011-0535-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0535-x