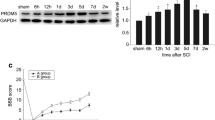
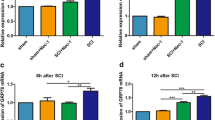
Abstract
Spinal cord injury (SCI) induces a series of endogenous biochemical changes that lead to secondary degeneration, including apoptosis. p53-mediated mitochondrial apoptosis is likely to be an important mechanism of cell death in spinal cord injury. However, the signaling cascades that are activated before DNA fragmentation have not yet been determined. DNA damage-induced, p53-activated neuronal cell death has already been identified in several neurodegenerative diseases. To determine DNA damage-induced, p53-mediated apoptosis in spinal cord injury, we performed RT-PCR microarray and analyzed 84 DNA damaging and apoptotic genes. Genes involved in DNA damage and apoptosis were upregulated whereas anti-apoptotic genes were downregulated in injured spinal cords. Western blot analysis showed the upregulation of DNA damage-inducing protein such as ATM, cell cycle checkpoint kinases, 8-hydroxy-2′-deoxyguanosine (8-OHdG), BRCA2 and H2AX in injured spinal cord tissues. Detection of phospho-H2AX in the nucleus and release of 8-OHdG in cytosol were demonstrated by immunohistochemistry. Expression of p53 was observed in the neurons, oligodendrocytes and astrocytes after spinal cord injury. Upregulation of phospho-p53, Bax and downregulation of Bcl2 were detected after spinal cord injury. Sub-cellular distribution of Bax and cytochrome c indicated mitochondrial-mediated apoptosis taking place after spinal cord injury. In addition, we carried out immunohistochemical analysis to confirm Bax translocation into the mitochondria and activated p53 at Ser392. Expression of APAF1, caspase 9 and caspase 3 activities confirmed the intrinsic apoptotic pathway after SCI. Activated p53 and Bax mitochondrial translocation were detected in injured spinal neurons. Taken together, the in vitro data strengthened the in vivo observations of DNA damage-induced p53-mediated mitochondrial apoptosis in the injured spinal cord.




Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ANOVA:
-
Analysis of variance
- APAF1:
-
Apoptotic protease activating factor 1
- ATM:
-
Ataxia telangiectasia mutated
- ATR:
-
Ataxia telangiectasia and Rad3 related
- BRCA2:
-
Breast cancer 2, early onset
- BSA:
-
Bovine serum albumin
- CHAPS:
-
3-[(3-Cholamidopropyl)dimethylammonio]propanesulfonic acid
- CHK2:
-
Checkpoint homolog (S. pombe)
- CNS:
-
Central nervous system
- DAB:
-
Diaminobenzidine
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- DNA:
-
Deoxyribonucleic acid
- DSB:
-
Double-strand breaks
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GFAP:
-
Glial fibrillary acidic protein
- H2AX:
-
Histone H2A.X
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HRP:
-
Horseradish peroxidase
- hUCB:
-
Human umbilical cord blood derived stem cells
- Olig2:
-
Oligodendrocyte transcription factor 2
- PAGE:
-
Poly acrylamide gel electrophoresis
- PBS:
-
Phosphate buffered saline
- RT-PCR:
-
Reverse Transcription based Polymerase chain reaction
- PMSF:
-
Phenyl methane sulfonyl fluoride
- SCI:
-
Spinal cord injury
- SDS:
-
Sodium dodecyl sulfate
- SSB:
-
Single-strand breaks
- STP:
-
Staurosporine
- TAE:
-
Tris-acetate-EDTA
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Hong LZ, Zhao XY, Zhang HL (2010) p53-mediated neuronal cell death in ischemic brain injury. Neurosci Bull 26:232–240
Love S (2003) Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry 27:267–282
Dasari VR, Veeravalli KK, Tsung AJ et al (2009) Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J Neurotrauma 26:2057–2069
McEwen ML, Springer JE (2005) A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem 53:809–819
Morrison RS, Kinoshita Y, Johnson MD et al (2003) p53-dependent cell death signaling in neurons. Neurochem Res 28:15–27
Vekrellis K, McCarthy MJ, Watson A et al (1997) Bax promotes neuronal cell death and is downregulated during the development of the nervous system. Development 124:1239–1249
Morrison RS, Kinoshita Y (2000) The role of p53 in neuronal cell death. Cell Death Differ 7:868–879
Eve DJ, Dennis JS, Citron BA (2007) Transcription factor p53 in degenerating spinal cords. Brain Res 1150:174–181
Thornborrow EC, Patel S, Mastropietro AE et al (2002) A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene 21:990–999
Gross A, Jockel J, Wei MC et al (1998) Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J 17:3878–3885
Hsu YT, Youle RJ (1997) Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272:13829–13834
Wolter KG, Hsu YT, Smith CL et al (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Desagher S, Osen-Sand A, Nichols A et al (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144:891–901
Nomura M, Shimizu S, Ito T et al (1999) Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res 59:5542–5548
Brewer GJ, Price PJ (1996) Viable cultured neurons in ambient carbon dioxide and hibernation storage for a month. Neuroreport 7:1509–1512
Lee SM, Yune TY, Kim SJ et al (2003) Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma 20:1017–1027
Liu XZ, Xu XM, Hu R et al (1997) Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 17:5395–5406
Dasari VR, Veeravalli KK, Saving KL et al (2008) Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol Dis 32:486–498
Giaccia AJ, Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev 12:2973–2983
Xiang H, Kinoshita Y, Knudson CM et al (1998) Bax involvement in p53-mediated neuronal cell death. J Neurosci 18:1363–1373
Chong MJ, Murray MR, Gosink EC et al (2000) Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system. Proc Natl Acad Sci USA 97:889–894
Johnson MD, Xiang H, London S et al (1998) Evidence for involvement of Bax and p53, but not caspases, in radiation-induced cell death of cultured postnatal hippocampal neurons. J Neurosci Res 54:721–733
Amar AP, Levy ML (1999) Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 44:1027–1039
Crowe MJ, Bresnahan JC, Shuman SL et al (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73–76
Schwab JM, Conrad S, Monnier PP et al (2005) Spinal cord injury-induced lesional expression of the repulsive guidance molecule (RGM). Eur J Neurosci 21:1569–1576
Olson MO (2004) Sensing cellular stress: another new function for the nucleolus? Sci STKE 2004:e10
Rubbi CP, Milner J (2003) Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J 22:6068–6077
Kalita K, Makonchuk D, Gomes C et al (2008) Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem 105:2286–2299
Parlato R, Kreiner G, Erdmann G et al (2008) Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J Neurosci 28:12759–12764
Keramaris E, Hirao A, Slack RS et al (2003) Ataxia telangiectasia-mutated protein can regulate p53 and neuronal death independent of Chk2 in response to DNA damage. J Biol Chem 278:37782–37789
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22:9030–9040
Slee EA, O’Connor DJ, Lu X (2004) To die or not to die: how does p53 decide? Oncogene 23:2809–2818
Cregan SP, MacLaurin JG, Craig CG et al (1999) Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci 19:7860–7869
Lowe SW, Ruley HE (1993) Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev 7:535–545
Manev H, Kharlamov A, Armstrong DM (1994) Photochemical brain injury in rats triggers DNA fragmentation, p53 and HSP72. Neuroreport 5:2661–2664
Shaw P, Bovey R, Tardy S et al (1992) Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA 89:4495–4499
Wyllie A (1997) Apoptosis. Clues in the p53 murder mystery. Nature 389:237–238
Sandig V, Brand K, Herwig S et al (1997) Adenovirally transferred p16INK4/CDKN2 and p53 genes cooperate to induce apoptotic tumor cell death. Nat Med 3:313–319
Ford JM, Hanawalt PC (1997) Role of DNA excision repair gene defects in the etiology of cancer. Curr Top Microbiol Immunol 221:47–70
Chen G, Branton PE, Shore GC (1995) Induction of p53-independent apoptosis by hygromycin B: suppression by Bcl-2 and adenovirus E1B 19-kDa protein. Exp Cell Res 221:55–59
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Vaux DL, Korsmeyer SJ (1999) Cell death in development. Cell 96:245–254
Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 3:614–620
Acknowledgments
We are thankful to Noorjehan Ali for technical assistance. We thank Shellee Abraham for manuscript preparation, and Sushma Jasti and Diana Meister for review of the manuscript. This study was funded by a grant from Caterpillar Inc., Peoria, IL and OSF Saint Francis Inc., Peoria, IL (J.S.R.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ramaprasada Rao Kotipatruni and Venkata Ramesh Dasari contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2011_530_MOESM1_ESM.tif
Supplementary Figure 1. Activation of p53 in spinal neurons in vitro . (A) Immunoblot analysis of p53 and phospho-p53 in STP (2µM)-treated spinal neurons. GAPDH was used as a control. (B) Quantitation of (A). Error bars indicate mean ± SE. Significant at P < 0.05. (C) Confocal scanning microscopic images demonstrate p53 expression in STP-treated spinal neurons. (D) Immunocytochemistry of phospho-p53 in STP-treated spinal neurons. DAPI was used for nuclear staining. Bar = 200µm. (TIFF 7831 kb)
11064_2011_530_MOESM2_ESM.tif
Supplementary Figure 2. Mitochondrial Bax-mediated apoptosis in injured spinal neurons. (A) Fluorescent microscopic images demonstrate p53 expression with Bax co-localization in STP-treated spinal neurons. Bax is conjugated with Alexa Fluor 488 secondary antibody and p53 is conjugated with Alexa Fluor 594 secondary antibodies. (B) Bax translocation into the mitochondria. Bax expression showed by green fluorescence and HSP60 used to recognize the mitochondrial location. Bax is conjugated with Alexa Fluor 488 secondary antibody and HSP60 is conjugated with Alexa Fluor 594 secondary antibodies. Bar = 200µm. n=3. (TIFF 6926 kb)
Rights and permissions
About this article
Cite this article
Kotipatruni, R.R., Dasari, V.R., Veeravalli, K.K. et al. p53- and Bax-Mediated Apoptosis in Injured Rat Spinal Cord. Neurochem Res 36, 2063–2074 (2011). https://doi.org/10.1007/s11064-011-0530-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0530-2