Abstract
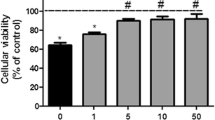
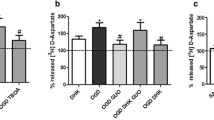
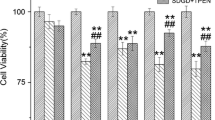
The adenine nucleotides ADP and ATP are probably the most important endogenous inhibitors of the mitochondrial permeability transition (MPT). We studied the inhibitory effects of adenine nucleotides on brain MPT by measuring mitochondrial swelling and Ca2+ and cytochrome c release. We observed that in the presence of either ADP or ATP, at 250 μM, brain mitochondria accumulated more than 1 μmol Ca2+ × mg protein−1. ADP or ATP also prevented Ca2+-induced mitochondrial swelling and cytochrome c release. Interestingly, ATP lost most of its inhibitory effects on MPT when the experiments were carried out in the presence of ATP-regenerating systems. These results indicate that MPT inhibition observed in the presence of added ATP could be mainly due to hydrolysis of ATP to ADP. From mitochondrial swelling measurements, half-maximal inhibitory values (K i) of 4.5 and 98 μM were obtained for ADP and ATP, respectively. In addition, a delayed mitochondrial swelling sensitive to higher ADP concentrations was observed. Mitochondrial anoxia/reoxygenation did not interfere with the inhibitory effect of ADP on Ca2+-induced MPT, but oxidative phosphorylation markedly decreased this effect. We conclude that ADP is a potent inhibitor of brain MPT whereas ATP is a weaker inhibitor of this phenomenon. Our results suggest that ADP can have an important protective role against MPT-mediated tissue damage under conditions of brain ischemia and hypoglycemia.






Similar content being viewed by others
References
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Kowaltowski AJ, Castilho RF, Vercesi AE (2001) Mitochondrial permeability transition and oxidative stress. FEBS Lett 495:12–15
Kim JS, He L, Lemasters JJ (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304:463–470
Albin RL, Greenamyre JT (1992) Alternative excitotoxic hypotheses. Neurology 42:733–738
Fiskum G, Murphy AN, Beal MF (1999) Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 19:351–369
Fernandes AM, Landeira-Fernandez AM, Souza-Santos P, Carvalho-Alves PC, Castilho RF (2008) Quinolinate-induced rat striatal excitotoxicity impairs endoplasmic reticulum Ca2+-ATPase function. Neurochem Res 33:1749–1758
Mirandola SR, Melo DR, Saito A, Castilho RF (2010) 3-nitropropionic acid-induced mitochondrial permeability transition: comparative study of mitochondria from different tissues and brain regions. J Neurosci Res 88:630–639
Nicholls DG, Budd SL (2000) Mitochondria and neuronal survival. Physiol Rev 80:315–360
Gunter TE, Sheu SS (2009) Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim Biophys Acta 1787:1291–1308
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343
Petronilli V, Cola C, Massari S, Colonna R, Bernardi P (1993) Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J Biol Chem 268:21939–21945
Halestrap AP, Woodfield KY, Connern CP (1997) Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem 272:3346–3354
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427:461–465
Sims NR (1990) Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55:698–707
Kristián T, Weatherby TM, Bates TE, Fiskum G (2002) Heterogeneity of the calcium-induced permeability transition in isolated non-synaptic brain mitochondria. J Neurochem 83:1297–1308
Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G (1996) Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA 93:9893–9898
Fornazari M, de Paula JG, Castilho RF, Kowaltowski AJ (2008) Redox properties of the adenoside triphosphate-sensitive K+ channel in brain mitochondria. J Neurosci Res 86:1548–1556
Fabiato A, Fabiato F (1978) Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. J Physiol 276:233–255
Andreyev AY, Fahy B, Fiskum G (1998) Cytochrome c release from brain mitochondria is independent of the mitochondrial permeability transition. FEBS Lett 439:373–376
Maciel EN, Kowaltowski AJ, Schwalm FD, Rodrigues JM, Souza DO, Vercesi AE, Wajner M, Castilho RF (2004) Mitochondrial permeability transition in neuronal damage promoted by Ca2+ and respiratory chain complex II inhibition. J Neurochem 90:1025–1035
Chudapongse P (1976) Further studies on the effect of phosphoenolpyruvate on respiration-dependent calcium transport by rat heart mitochondria. Biochim Biophys Acta 423:196–202
Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR (1996) The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol 496:111–128
Uchino H, Elmér E, Uchino K, Li PA, He QP, Smith ML, Siesjö BK (1998) Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res 812:216–226
Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T (1999) Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 19:736–741
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010
Liu RR, Murphy TH (2009) Reversible cyclosporine A sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo, a two-photon imaging study. J Biol Chem 284:36109–36117
Nicholls DG, Scott ID (1980) The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem J 186:833–839
Kristián T, Pivovarova NB, Fiskum G, Andrews SB (2007) Calcium-induced precipitate formation in brain mitochondria: composition, calcium capacity, and retention. J Neurochem 102:1346–1356
Maciel EN, Kaminski Schierle GS, Hansson O, Brundin P, Castilho RF (2003) Cyclosporin A and Bcl-2 do not inhibit quinolinic acid-induced striatal excitotoxicity in rodents. Exp Neurol 183:430–437
Vergun O, Reynolds IJ (2005) Distinct characteristics of Ca2+-induced depolarization of isolated brain and liver mitochondria. Biochim Biophys Acta 1709:127–137
Shalbuyeva N, Brustovetsky T, Brustovetsky N (2007) Lithium desensitizes brain mitochondria to calcium, antagonizes permeability transition, and diminishes cytochrome c release. J Biol Chem 282:18057–18068
Rottenberg H, Marbach M (1989) Adenine nucleotides regulate Ca2+ transport in brain mitochondria. FEBS Lett 247:483–486
Rottenberg H, Marbach M (1990) Regulation of Ca2+ transport in brain mitochondria. II. The mechanism of the adenine nucleotides enhancement of Ca2+ uptake and retention. Biochim Biophys Acta 1016:87–98
Broekemeier KM, Pfeiffer DR (1989) Cyclosporin A-sensitive and insensitive mechanisms produce the permeability transition in mitochondria. Biochem Biophys Res Commun 163:561–566
Maciel EN, Vercesi AE, Castilho RF (2001) Oxidative stress in Ca2+-induced membrane permeability transition in brain mitochondria. J Neurochem 79:1237–1245
Williams SP, Fulton AM, Brindle KM (1993) Estimation of the intracellular free ADP concentration by 19F NMR studies of fluorine-labeled yeast phosphoglycerate kinase in vivo. Biochemistry 32:4895–4902
Vercesi AE, Kowaltowski AJ, Oliveira HC, Castilho RF (2006) Mitochondrial Ca2+ transport, permeability transition and oxidative stress in cell death: implications in cardiotoxicity, neurodegeneration and dyslipidemias. Front Biosci 11:2554–2564
Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T (1998) Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci 18:5151–5159
Ha HC, Snyder SH (2000) Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol Dis 7:225–239
Alano CC, Ying W, Swanson RA (2004) Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem 279:18895–18902
Acknowledgments
The authors would like to thank Edilene S. Santos for her technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Instituto Nacional de Obesidade e Diabetes. A.S. was supported by a FAPESP fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, Â., Castilho, R.F. Inhibitory Effects of Adenine Nucleotides on Brain Mitochondrial Permeability Transition. Neurochem Res 35, 1667–1674 (2010). https://doi.org/10.1007/s11064-010-0228-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0228-x