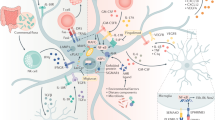
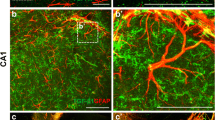
Abstract
Flavonoids are naturally occurring polyphenolic compounds that are present in a variety of fruits, vegetables, cereals, tea, and wine, and are the most abundant antioxidants in the human diet. Evidence suggests that these phytochemicals might have an impact on brain pathology and aging; however, neither their mechanisms of action nor their cell targets are completely known. In the mature mammalian brain, astroglia constitute nearly half of the total cells, providing structural, metabolic, and trophic support for neurons. During the past few years, increasing knowledge of these cells has indicated that astrocytes are pivotal characters in neurodegenerative diseases and brain injury. Most of the physiological benefits of flavonoids are generally thought to be due to their antioxidant and free-radical scavenging effects; however, emerging evidence has supported the hypothesis that their mechanism of action might go beyond these properties. In this review, we focus on astrocytes as targets for flavonoids and their implications in brain development, neuroprotection, and glial tumor formation. Finally, we will briefly discuss the emerging view of astrocytes as essential characters in neurodegenerative diseases, and how a better understanding of the action of flavonoids might open new avenues to develop therapeutic approaches to these pathologies.



Similar content being viewed by others
References
Virchow, 1846, quoted from Somjen GG (1988) Nervenkitt: notes on the history of the concept of neuroglia. Glia 1: 2–9
Kettenmann H, Ransom BR (2005) Neuroglia, 2nd edn. Oxford University Press, USA
Lim DA, Alvarez‐Buylla A (1999) Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA 96:7526–7531
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Lie DC, Colamarino SA, Song HJ et al (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375
de Sampaio e Spohr TCL, Choi JW, Gardell SE et al (2008) Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem 283:7470–7479
Hatten ME (2002) New directions in neuronal migration. Science 297:1660–1663
Garcia-Abreu J, Moura-Neto V, Carvalho SL et al (1995) Regionally specific properties of midbrain glia: I. Interactions with midbrain neurons. J Neurosci Res 40:417–477
Martinez R, Gomes FCA (2002) Neuritogenesis induced by thyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen‐activated protein kinase‐phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem 51:49311–49318
Martinez R, Gomes FC (2005) Proliferation of cerebellar neurons induced by astrocytes treated with thyroid hormone is mediated by a cooperation between cell contact and soluble factors and involves the epidermal growth factor‐protein kinase a pathway. J Neurosci Res 3:341–349
Christopherson KS, Ullian EM, Stokes CC et al (2005) Thrombospondins are astrocyte secreted proteins that promote CNS synaptogenesis. Cell 120:421–433
Stevens B, Allen NJ, Vazquez LE et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 6:1164–1178
Gomes FCA, Garcia-Abreu J, Galou M et al (1999) Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. Glia 26:97–108
de Sampaio e Spohr TCL, Martinez R, Federowicz ES et al (2002) Neuron-glia interaction effects on GFAP gene: a novel role for transforming growth factor-β1. Eur J Neurosci 16:2059–2069
Schmid RS, McGrath B, Berechid BE et al (2003) Neuregulin1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Neuroscience 100:4251–4256
Sousa VO, Romão L, Moura Neto V et al (2004) Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur J Neurosci 19:1721–1730
Barnabé-Heider F, Wasylnka JA, Fernandes KJL et al (2005) Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48:253–265
Stipursky J, Gomes FCA (2007) TGF-β1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55:1023–1033
Noctor SC, Flint AC, Weissman TA et al (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714–720
Alvarez-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41:683–686
Stipursky J, de Sampaio e Spohr TCL, Romão L, Gomes FCA (2010) Neuron-astrocyte interaction in cell fate commitment in the central nervous system. In: Stem cells: from tools for studying mechanism of neuronal differentiation towards therapy, vol 11. Springer, pp 145–170
Cahoy J, Emery B, Kaushal A et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278
Vesce S, Bezzi P, Volterra A (1999) The active role of astrocytes in synaptic transmission. Cell Mol Life Sci 56:991–1000
Landis DM (1994) The early reactions of non-neuronal cells to brain injury. Annu Rev Neurosci 17:133–151
Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638
Kim JH, Kim JH, Park JA (2006) Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol 39:339–345
Chamak B, Prochiantz A (1989) Influence of extracellular matrix proteins on the expression of neuronal polarity. Development 3:483–491
Gomes FC, de Sampaio e Spohr TC, Martinez R, Moura Neto V (2001) Cross-talk between neurons and glia: highlights on soluble factors. Braz J Med Biol Res 34:611–620
Alvarez-Buylla A, Garcia-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22:629–634
Goldman SA, Nottebohm F (1983) Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA 80:2390–2394
Doetsch F, Caille I, Lim DA (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716
Seri B, García-Verdugo JM, McEwen BS et al (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160
Bushong EA, Martone ME, Jones YZ et al (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 1:183–192
Araque A, Parpura V, Sanzgiri RP et al (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 5:208–215
Pfrieger FW, Barres BA (1997) Synaptic efficacy enhanced by glial cells in vitro. Science 277:1684–1687
Ullian EM, Christopherson KS, Barres BA (2004) Role for glia in synaptogenesis. Glia 47:209–216
Ullian EM, Sapperstein SK, Christopherson KS (2001) Control of synapse number by glia. Science 291:657–661
Nägler K, Mauch DH, Pfrieger FW (2001) Glia‐derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol 533:665–679
Johnson MA, Weick JP, Pearce RA (2007) Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 12:3069–3077
Parpura V, Haydon PG (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 15:8629–8634
Goritz C, Mauch D, Pfrieger FW (2005) Multiple mechanisms mediate cholesterol induced synaptogenesis in a CNS neuron. Mol Cell Neurosci 29:190–201
Feng Z, Ko CP (2008) Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor‐beta1. J Neurosci 39:9599–9609
Koizumi S, Fujishita K, Tsuda M (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte‐derived ATP in hippocampal cultures. Proc Natl Acad Sci USA 19:11023–11028
Yang KW, Lee KW, Kim SH (2006) The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett 395:103–107
Nishiyama H, Knopfel T, Endo S (2002) Glial protein S100β modulates long‐term neuronal synaptic plasticity. Proc Natl Acad Sci USA 99:4037–4042
Bezzi P, Volterra A (2001) A neuron‐glia signalling network in the active brain. Curr Opin Neurobiol 11:387–394
Wilhelmsson U, Bushong EA, Price DL et al (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A 103:17513–17518
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36:180–190
Ridet JL, Malhotra SK, Privat A et al (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:570–577
Sawada M, Kondo N, Suzumura A et al (1989) Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res 491:394–397
Becher B, Prat A, Antel JP (2000) Brain-immune connection: Immuno regulatory properties of CNS resident cells. Glia 29:293–304
Dietrich PY, Walker PR, Saas P (2003) Death receptors on reactive astrocytes: a key role in the fine tuning of brain inflammation? Neurology 60:548–554
Gerlach M, Double KL, Ben-Shachar D et al (2003) Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson’s disease. Neurotoxicol Res 5:35–44
Schipper HM (1996) Astrocytes, brain aging, and neurodegeneration. Neurobiology 17:467–480
Minghetti L, Levi G (1998) Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog Neurobiol 54:99–125
Minghetti L, Levi G (1998) Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog Neurobiol 54:99–125
Gonzalez-Scarano F, Baltuch G (1999) Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22:219–240
Barbeito LH, Pehar M, Cassina P (2004) A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev 47:263–274
DeWitt DA (1998) Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer’s disease. Exp Neurol 149:329–340
Wisniewski HM, Wegiel J (1991) Spatial relationships between astrocytes and classical plaque components. Neurobiol Aging 12:593–600
Nagele RG (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674
Nutt JG, Wooten GF (2005) Clinical practice: diagnosis and initial management of Parkinson’s disease. N Engl J Med 353:1021–1027
Klegeris A, McGeer EG, McGeer PL (2007) Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol 20:351–357
Forno LS (1992) Astrocytes and Parkinson’s disease. Prog Brain Res 94:429–436
Mena MA (2008) Glial cells as players in parkinsonism: the “good”, the “bad”, and the “mysterious” glia. Neuroscientist 14:544–560
Bruijn LI, Becher MW, Lee MK (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338
Howland DS (2002) Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA 99:1604–1609
Patel SA, Maragakis NJ (2002) Amyotrophic lateral sclerosis: pathogenesis, differential diagnoses, and potential interventions. J Spinal Cord Med 25:262–273
Kleihues P, Louis DN, Scheithauer BW (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Brandes AA, Tosoni A, Franceschi E (2008) Glioblastoma in adults. Crit Rev Oncol Hematol 67:139–152
Shih AH, Holland EC (2004) Developmental neurobiology and the origin of brain tumors. J Neurooncol 70:125–136
Louis DN, Holland EC, Cairncross JG (2001) Glioma classification: a molecular reappraisal. Am J Pathol 159:779–786
Wild-Bode C, Weller M, Rimner A et al (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61:2744–2750
Huse JT, Holland EC (2009) Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol 19:132–143
Bent MJ, Hegi ME, Stupp R (2006) Recent developments in the use of chemotherapy in brain tumours. Eur J Cancer 42:582–588
Chambaut-Guérin AM, Costa SL, Lefrançois T et al (2000) Effects of retinoic acid and tumor necrosis factor alpha on GL-15 glioblastoma cells. Neuronreporter 11:389–393
Costa SL, Paillaud E, Fages C (2001) Effects of a novel synthetic retinoid on malignant glioma in vitro: inhibition of cell proliferation, induction of apoptosis and differentiation. Eur J Cancer 37:520–530
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Gerstner ER, Duda DG, Di Tomaso E et al (2007) Antiangiogenic agents for the treatment of glioblastoma. Expert Opin Investig Drugs 16:1895–1908
Brandsma D, Stalpers L, Taal W et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Feldmann KA (2001) Cytochrome P450s as genes for crop improvement. Curr Opin Plant Biol 4:162–167
Beecher GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 133:3248S–3254S
Harborne JB (1986) Nature, distribution and function of plant flavonoids. Prog Clin Biol Res 213:15–24
Manach C, Scalbert A, Morand C et al (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Wang Y, Ho CT (2009) Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem 57:8109–8114
Hou L, Zhou B, Yang L et al (2004) Inhibition of human low density lipoprotein oxidation by flavonols and their glycosides. Chem Phys Lipids 129:209–219
Halliwell B (1998) Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions and preliminary results from nutritional supplementation studies. Free Radic Res 6:469–486
Williams RJ, Spencer JPE, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36:838–849
Spencer JPE, Rice-Evans C, Williams RJ (2003) Modulation of pro-survival Akt/PKB & ERK1/2 signalling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 278:34783–34793
Galli RL, Shukitt-Hale B, Youdim KA (2002) Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Ann N Y Acad Sci 959:128–132
Unno K, Takabayashi F, Kishido T (2004) Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence. Exp Gerontol 39:1027–1034
Haque AM, Hashimoto M, Katakura M et al (2006) Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr 136:1043–1047
Kuriyama S, Hozawa A, Ohmori K (2006) Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project. Am J Clin Nutr 83:355–361
Wang Y, Wang L, Wu J et al (2006) The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol 148:147–153
Rice-Evans C (2001) Flavonoid antioxidants. Curr Med Chem 8:797–807
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Abd El Mohsen MM, Kuhnle G, Rechner AR (2002) Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 33:1693–1702
Schroeter H, Spencer JPE, Rice-Evans C (2001) Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J 358:547–557
Youdim KA, Qaiser MZ, Begley DJ (2004) Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 36:592–604
Silva AR, Pinheiro AM, Souza CS et al (2008) The flavonoid rutin induces astrocyte and microglia activation and regulates TNF-alpha and NO release in primary glial cell cultures. Cell Biol Toxicol 24:75–86
Bahia PK, Rattray M, Williams RJ (2008) Dietary flavonoid (-)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J Neurochem 106:2194–2204
Wu BY, Yu AC (2000) Quercetin inhibits c-fos, heat shock protein, and glial fibrillary acidic protein expression in injured astrocytes. J Neurosci Res 62:730–736
Gitika B, Sai Ram M, Sharma SK (2006) Quercetin protects C6 glial cells from oxidative stress induced by tertiary-butylhydroperoxide. Free Radic Res 40:95–102
Sharma V, Mishra M, Ghosh S et al (2007) Modulation of interleukin-1 mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull 73:55–63
Almeida LMV, Pinheiro CC, Leite MC et al (2008) Protective effects of resveratrol. toxicity in primary cortical astrocyte. Neurochem Res 33:8–15
de Sampaio e de Sampaio e Spohr TC, Stipursky J, Sasaki AC et al (2010) Effects of the flavonoid casticin from Brazilian Croton betulaster in cerebral cortical progenitors in vitro: direct and indirect action through astrocytes. J Neurosci Res 88:530–541
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34
Singh M, Arseneault M, Sanderson T et al (2008) Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem 56:4855–4873
Scheck AC, Perry K, Hank NC (2006) Anticancer activity of extracts derived from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells. BMC Comp Alt Med 6:1–9
Ferguson PJ, Kurowska EM, Freeman DJ et al (2006) In vivo inhibition of growth on human tumor lines by flavonoid fractions from cranberry extract. J In Nutri Cancer 56:86–94
Braganhol E, Zamin LL, Canedo AD et al (2006) Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs 17(6):663–671
Sharma V, Joseph C, Ghosh S (2007) Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther 6:2544–2553
Son YG, Kim EH, Kim JY (2007) Silibinin sensitizes human glioma cells to TRAIL-Mediated Apoptosis via DR5 Up-regulation and Down-regulation of c-FLIP and Survivin. Cancer Res 67:8274–8284
Amado NG, Cerqueira DM, Menezes FS (2009) Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs 20:543–552
Barbosa PR, Martins D, Fascio M et al (2004) Benzoyl-methylpolyols from Croton species (Euphorbiaceae). Arkivoc 6:95–102
Stoclet JC, Chataigneau T, Ndiaye M et al (2004) Vascular protection by dietary polyphenols. Eur J Pharmacol 500:299–313
Duthie SJ (2007) Berry phytochemicals, genomic stability and cancer: evidence for chemoprotection at several stages in the carcinogenic process. Mol Nutr Food Res 51:665–674
Manach C, Mazur A, Scalbert A (2005) Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol 16:77–84
Vauzour D, Vafeiadou K, Rodriguez-Mateos A et al (2008) The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 3:115–126
De Rijk MC, Breteler MM, den Breeijen JH et al (1997) Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch Neurol 54:762–765
Engelhart MJ, Geerlings MI, Ruitenberg A (2002) Dietary intake of antioxidants and risk of Alzheimer disease. AMA 26:3223–3229
Hellenbrand W, Seidler A, Boeing H et al (1996) Diet and Parkinson’s disease. I: A possible role for the past intake of specific foods and food groups. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 47:636–643
Costa BL, Fawcett R, Li GY et al (2008) Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull 1:412–423
Lau FC, Shukitt-Hale B, Joseph JA (2005) The beneficial effects of fruit polyphenols on brain aging. Biochem Pharmacol 5:339–345
Rainey-Smith S, Schroetke LW, Bahia P (2008) Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci Lett 438:29–33
Mandel S, Maor G, Youdim MB (2004) Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (-)-epigallocatechin-3-gallate. J Mol Neurosci 24:401–416
Simonyi A, Wang Q, Miller RL et al (2005) Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31:135–147
Nijveldt RJ, van Nood E, van Hoorn DE (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Schwarting RK, Huston JP (1997) Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology 18:689–708
Levites Y, Weinreb O, Maor G et al (2001) Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 78:1073–1082
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 280:37377–37382
Rezai-Zadeh K, Shytle D, Sun N (2005) Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25:8807–88014
Bastianetto S, Yao ZX, Papadopoulos V et al (2006) Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur J Neurosci 23:55–64
Choi YT, Jung CH, Lee SR (2001) The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci 70:603–614
Oken BS, Storzbach DM, Kaye JA (1998) The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 55:1409–1415
Obregon DF, Rezai-Zadeh K, Bai Y et al (2006) ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem 281:16419–16427
Weinreb O, Mandel S, Amit T et al (2004) Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutr Biochem 15:506–516
Zafrilla P, Morillas JM, Rubio-Perez JM et al (2009) Ingredients for functional drinks in neurodegenerative diseases: a review. Nat Prod Commun 4:719–740
Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 11:1355–1360
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nature Clinical Practice 12:679–689
Marchetto MC, Muotri AR, Mu Y (2008) Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3:649–657
Xu Z, Chen S, Li X et al (2006) Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res 10:1263–1269
Acknowledgments
We thank Dr. Janet W. Reid, JWR Associates, Biological Consulting and Editing Services for revising the English text. This study was supported by grants from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nones, J., Stipursky, J., Costa, S.L. et al. Flavonoids and Astrocytes Crosstalking: Implications for Brain Development and Pathology. Neurochem Res 35, 955–966 (2010). https://doi.org/10.1007/s11064-010-0144-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0144-0