Abstract
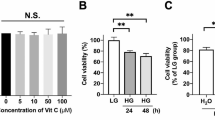
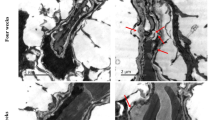
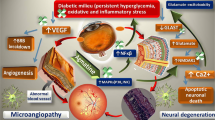
Diabetes-induced increase in oxidative stress is postulated as playing a significant role in the development of retinopathy. The retinal pigment epithelium (RPE) which forms part of the retinal blood barrier has been reported to be affected in diabetes. Besides functioning as a neurotransmitter, the radical nitric oxide (NO) can act as a cytotoxic agent. NO is synthesized by nitric oxide synthase (NOS) that oxidizes arginine to citrulline producing NO. Given that intracellular concentration of arginine depends mainly on its transport, we studied arginine transport in RPE and retina from normal and streptozotocin-induced diabetic rats. Retina and RPE take up arginine by a saturable system with an apparent KM of 70–80 μM. Tissue incubation in the presence of insulin or high glucose concentrations significantly increased arginine transport in RPE but not in retina from control rats. Similarly, arginine uptake was enhanced in RPE, but not in the retina from streptozotocin-induced diabetic rats. However, NO content was two-fold higher in diabetic retina and RPE compared to that in the control rats. Such findings may suggest that diabetes induced an increase in NO levels in RPE, which may have brought about alterations in its functioning and in turn manifestations of diabetic retinopathy.




Similar content being viewed by others
References
Frank RN (1995) Diabetic retinopathy. Prog Retin Eye Res 14:361–392
McGregor LC, Rosecan LR, Laties AM, Matschinsky FM (1986) Altered retinal metabolism in diabetes. J Biol Chem 261:4046–4051
Kowluru RA, Tang J, Kern TS (2001) Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50:1938–1942
Armstrong D, Abdella N, Salman A, Miller N, Rahman EA, Bojancyzk M (1992) Relationship of lipid peroxides to diabetic complications. J Diabetes Complications 6:116–122
Jennings PE, McLaren M, Scott NA, Saniabadi AF, Belch JJF (1991) The relationship of oxidative stress to thrombotic tendency in type 1 diabetic patients with retinopathy. Diabetic Med 8:860–865
Low PA, Nickander KK, Tritschler HJ (1997) The roles of oxidative stress and antioxidant treatment in diabetic neuropathy. Diabetes 46:530–542
Bayns JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective of an old paradigm. Diabetes 48:1–9
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neuronal role for nitric oxide. Nature 347:768–770
Bredt DS, Snyder SH (1992) Nitric oxide, a novel neuronal messenger. Neuron 8:3–11
Salgo MG, Bermudez E, Squadrito GL, Pryor WA (1995) DNA damage and oxidation of thiols, peroxinitrite causes in rat thymocytes. Arch Biochem Biophys 322:500–505
White MF (1985) The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochem Biophys Acta 822:355–374
Mac Leod CL, Finley KD, Kakuda DK (1994) y+ type cationic amino acid transport: expression and regulation of the m Cat genes. J Exp Biol 196:109–121
Kirber WM, Nichols CW, Grimes PA, Winegard AI, Laties AM (1980) A permeability defect of the retinal pigment epithelium. Arch Opthalmol 98:725–728
Vinores SA, Campochiaro PA (1989) Prevention or moderation of some ultrastructural changes on the RPE and retina of galactosemic rat by aldose reductase inhibition. Exp Eye Res 49:495–510
Vilchis C, Salceda R (1996) Effect of diabetes on levels and uptake of putative amino acid neurotransmitters in rat retina and retinal pigment epithelium. Neurochem Res 21:1167–1171
Salceda R (1989) Uptake and K+-stimulated release of 14C-glycine from frog retinal synaptosomal fractions. Neurochem Res 14:49–54
Ignarro LJ (1990) Nitic oxide. A novel signal transduction mechanism for transcellular communication. Hypertension 16:477–483
Du Y, Smith MA, Miller CM, Kern TS (2002) Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem 80:771–779
Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC (1999) l-Arginine transport in retinas from streptozotocin diabetic rats: correlation with the level of IL-1β and NO synthase activity. Vision Res 39:3817–3823
Kawano T, Nomura M, Nisikado A, Nakaya Y, Ito S (2003) Supplementation of l-arginine improves hypertension and lipid metabolism but not Insulin resistance in diabetic rats. Life Sci 73:3017–3026
Bogle RG, Baydoun AR, Pearson JD, Mann GE (1996) Regulation of l-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol 490:229–241
Wu F, Cholewa B, Mattson DL (2000) Characterization of l-arginine transporters in rat renal inner medullary collecting duct. Am J Physiol Regulatory Integrative Comp Physiol 278:R1506–R1512
Westergaard N, Beart PM, Schousboe A (1993) Transport of l-[3H] arginine in cultured neurons: characteristics and inhibition by nitric oxide synthase inhibitors. J Neurochem 61:364–367
Sobrevia L, Nadal A, Yudilevich DL, Mann GE (1996) Activation of l-arginine transport (system y+) and nitric oxide synthase by elevated glucose and insulin in human endothelial cells. J Physiol 490:775–781
Aldridge CR, Collard KJ (1996) The characteristics of arginine transport by rat cerebellar and cortical synaptosomes. Neurochem Res 21:1539–1546
Sáenz DA, Cymeryng CB, De Nichilo A, Sacca GB, Sarmiento MIK, Rosenstein RE (2002) Photic regulation of l-arginine uptake in the golden hamster retina. J Neurochem 80:512–519
Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384:145–155
Machado VL, Wassermann GF, Marques M (1991) In vitro effect of insulin on the uptake of glucose and alpha amino isobutyric acid in the thyroid gland of the turtle (Chrysemys dorbigni). Gen Comp Endocrinol 82:8–13
Muñoz M, Sweiry JH, Mann GE (1995) Insulin stimulates cationic amino acid transport activity in the isolated perfused rat pancreas. Exp Physiol 80:745–753
Salceda R (1999) Insulin-stimulated taurine uptake in rat retina and retinal pigment epithelium. Neurochem Int 35:301–306
Gu S, Villegas CJ, Jiang JX (2005) Differential regulation of amino acid transporter SNAT3 by insulin in hepatocytes. J Biol Chem 280:26055–26062
Handlogten ME, Kilberg MS (1984) Induction and decay of amino acid transport in liver. Turnover of transport activity in isolated hepatocytes after stimulation by diabetes or glucagon. J Biol Chem 259:3519–3525
Wu G, Flynn NE (1993) The activation of the arginine-citrulline cycle in macrophages from the spontaneously diabetic BB rat. Biochem J 294:113–118
Sobrevia L, Cesare P, Yudilevich DL, Mann GE (1995) Diabetes-induced activation of systems y+ and nitric oxide synthase in human endothelium cells: association with membrane hyperpolarization. J Physiol 489:183–192
Sobrevia L, Yudilevich DL, Mann GE (1998) Elevated d-glucose induces insulin sensitivity in human umbilical endothelial cells isolated from gestational diabetic pregnancies. J Physiol 506:219–230
Wu JY, Robinson D, Kung HJ, Hatzorglou M (1994) Hormonal regulation of the gene for the type C ecotropic retrovirus receptor in rat liver cells. J Virol 68:1615–1623
Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45:675–684
Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, Saskia J, Kim M, Melia M, Fishman M, Huang P, Campochiaro PA (2002) Nitric oxide is proangiogenic in the retina and choroids. J Cell Physiol 191:116–124
Acknowledgments
This study was partially supported by CONACYT grant U45840 and PAPIIT/UNAM grant IN 204000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Ricardo Tapia.
Rights and permissions
About this article
Cite this article
Salceda, R., Hernández-Espinosa, C. & Sánchez-Chávez, G. l-Arginine Uptake in Normal and Diabetic Rat Retina and Retinal Pigment Epithelium. Neurochem Res 33, 1541–1545 (2008). https://doi.org/10.1007/s11064-008-9641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9641-9