Summary
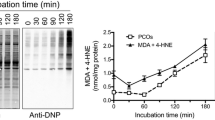
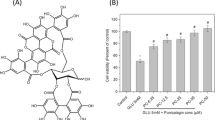
In this study, we investigated the possible link between lipid peroxidation (LPO) and the formation of protein carbonyls (PCOs) during depletion of brain glutathione (GSH). To this end, rat brain slices were incubated with the GSH depletor diethyl maleate (DEM) in the absence or presence of classical LPO scavengers: trolox, caffeic acid phenethyl ester (CAPE), and butylated hydroxytoluene (BHT). All three scavengers reduced DEM-induced lipid oxidation and protein carbonylation, suggesting that intermediates/products of the LPO pathway such as lipid hydroperoxides, 4-hydroxynonenal and/or malondialdehyde are involved in the process. Additional in vitro experiments revealed that, among these products, lipid hydroperoxides are most likely responsible for protein oxidation. Interestingly, BHT prevented the carbonylation of cytoskeletal proteins but not that of soluble proteins, suggesting the existence of different mechanisms of PCO formation during GSH depletion. In pull-down experiments, β-actin and α/β-tubulin were identified as major carbonylation targets during GSH depletion, although other cytoskeletal proteins such as neurofilament proteins and glial fibrillary acidic protein were also carbonylated. These findings may be important in the context of neurological disorders that exhibit decreased GSH levels and increased protein carbonylation such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis.




Similar content being viewed by others
Abbreviations
- AG:
-
Aminoguanidine
- BHT:
-
Butylated hydroxytoluene
- CAPE:
-
Caffeic acid phenethyl ester
- CNS:
-
Central nervous system
- DEM:
-
Diethyl maleate
- ECL:
-
Enhanced chemioluminescence
- GFAP:
-
Glial fibrillary acidic protein
- GSH:
-
Glutathione
- 4-HNE:
-
4-hydoxy-2-nonenal
- 15-HPETE:
-
15-hydroperoxy-5,8,11,13-eicosanotetraenoic acid
- Hy:
-
Hydralazine
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- NFH:
-
Neurofilament heavy (200 kDa) protein
- NFL:
-
Neurofilament light (69 kDa) protein
- NFM:
-
Neurofilament medium (150 kDa) protein
- PCO:
-
Protein carbonyl
- RCO:
-
Reactive carbonyl
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TBARS:
-
Thiobarbituric acid reactive substances
References
Stadtman ER, Berlett BS (1997) Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol 10:485–494
Levine RL (2002) Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med 32:790–796
Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR (2001) Protein oxidation in the brain in Alzheimer’s disease. Neuroscience 103:373–383
Floor E, Wetzel MG (1998) Increased protein oxidation in human sustantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem 70:268–275
Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Beal MF (1997) Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem 69:2064–2074
Bizzozero OA, Dejesus G, Callahan K, Pastuszyn A (2005) Elevated protein carbonylation in the brain white matter and gray matter of patients with multiple sclerosis. J Neurosci Res 81:687–695
Adams S, Green P, Claxton R, Simcox S, Williams MV, Walsh K, Leeuwenburgh C (2001). Reactive carbonyl formation by oxidative and non-oxidative pathways. Front Biosci 6:17–24
Fu SL, Dean R (1997) Structural characterization of the products of hydroxyl-radical damage to leucine and their detection on proteins. Biochem J 324:41–48
Sayre LM, Moreira PI, Smith MA, Perry G (2005) Metal ions and oxidative protein modification in neurological disease. Ann Ist Super Sanita 41:143–164
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR (1996) The advanced glycation end product, N-ε-(carboxymethyl)lysine is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 271:9982–9986
Refsgaard HF, Tsai L, Stadman ER (2000) Modification of proteins by polyunsaturated fatty acid peroxidation products. Proc Natl Acad Sci USA 97:611–691
Requena JS, Chao CC, Levine R, Stadtman ER (2001) Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci USA 98:69–74
Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. J Biol Chem 280:21522–21530
Yan LJ, Lodge JK, Traber MG, Packer L (1997) Apolipoprotein B carbonyl formation is enhanced by lipid peroxidation during copper-mediated oxidation of human low-density lipoproteins. Arch Biochem Biophys 339:165–171
Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem 68:2092–2097
Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y (1996) Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA 93:2696–2701
Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG, Kasarskis E, Mattson MP (1998) Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol 44:819–824
Bizzozero OA, Ziegler JL, De Jesus G, Bolognani F (2006) Acute depletion of reduced glutathione causes extensive carbonylation of rat brain proteins. J Neurosci Res 83:656–667
Riddles PW, Blakely RL, Zerner B (1979) Ellman’s reagent: 5,5′-dithiobis(2-nitrobenzoic acid)-a reexamination. Anal Biochem 94:75–81
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Amici A, Levine RL, Tsai L, Stadtman ER (1989) Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem 264:3341–3346
Buchmuller-Rouiller Y, Corrandin SB, Smith J, Schneider P, Ransijn A, Jongeneel CV, Mauel J (1995) Role of glutathione in macrophage activation: effect of cellular glutathione depletion on nitrite production and leishmanicidal activity. Cell Immunol 164:73–80
Kaminskas LM, Pyke SM, Burcham PC (2004) Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther 310:1003–1010
Al-Abed Y, Bucala R (1997) Efficient scavenging of fatty acid oxidation products by aminoguanidine. Chem Res Toxicol 10:875–879
Daiber A, Mülsch A, Hink U, Mollnau H, Warnholtz A, Oelze M, Münzel T (2005) The oxidative stress concept of nitrate tolerance and the antioxidant properties of hydralazine. Am J Cardiol 96:25–36
Giardino I, Fard AK, Hatchell DL, Brownlee M (1998) Aminoguanidine inhibits reactive oxygen species formation, lipid peroxidation, and oxidant-induced apoptosis. Diabetes 47:1114–1120
Chaiyasit W, McClements J, Decker EA (2005) The relationship between the physicochemical properties of antioxidants and their ability to inhibit lipid oxidation in bulk oil and oil–water emulsions. J Agric Food Chem 53:4982–4988
Son S, Lewis BA (2002) Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J Agric Food Chem 50:468–472
Pluta RM, Jung CS, Harvey-White J, Whitehead A, Shilad S, Espey MG, Oldfield EH (2005) In vitro and in vivo effects of probucol on hydrolysis of asymmetric dimethyl l-arginine and vasospasm in primates. J Neurosurg 103:731–738
Davies MJ, Slater TF (1987) Studies on the metal-ion and lipoxygenase-catalysed breakdown of hydroperoxides using electron-spin-resonance spectroscopy. Biochem J 245:167–173
Pennathur S, IdoY, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW (2005) Reactive carbonyls and polyunsaturated fatty acids produce hydroxyl radical-like species. J Biol Chem 280:22706–22714
Muntané G, Dalfó E, Martínez A, Rey MJ, Avila J, Pérez M, Portero M, Pamplona R, Ayala V, Ferrer I (2006) Glial fibrillary acidic protein is a major target of glycoxidative and lipoxidative damage in Pick’s disease. J Neurochem 99:177–185
Butterfield DA, Abdul HM, Newman S, Reed T (2006) Redox proteomics in some age-related neurodegenerative disorders or models thereof. NeuroRx 3:344–357
Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A (2004) Novel effect of NF-κB activation: carbonylation and nitration injury to cytoskeleton and disruption of monolayer barrier in intestinal epithelium. Am J Physiol Cell Physiol 287:C139–C151
Neely MD, Boutte A, Milatovic D, Montine TJ (2005) Mechanisms of 4-hydroxynonenal-induced neuronal microtubule dysfunction. Brain Res 1037:90–98
Dalle-Done I, Rossi R, Giustarini D, Gagliano N, Lusini L, Milzani A, Di Simplicio P, Colombo R (2001) Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic Biol Med 31:1075–1083
Ozeki M, Miyagawa-Hayashino A, Akatsuka S, Shirase T, Lee WH, Uchida K, Toyokuni S (2005) Susceptibility of actin to modification by 4-hydroxy-2-nonenal. J Chromatogr 827:119–126
Banan A, Fitzpatrick L, Zhang LJ, Keshavarzian A (2001) OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic Biol Med 30:287–298
Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PL, Shimohama S, Szweda LI, Kaminski MA, Avila J, Price DL, Cleveland DW, Sayre LM, Perry G (2002) High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem 277:4644–4648
Gelinas S, Chapados C, Beauregard M, Gosselin I, Martinoli MG (2000) Effect of oxidative stress on stability and structure of neurofilament proteins. Biochem Cell Biol 78:667–674
Smith MA, Rudnicka-Nawrot M, Richey PL, Praprotnik D, Mulvihill P, Miller CA, Sayre LM, Perry G (1995) Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer’s disease. J Neurochem 64:2660–2666
Toncoso JC, Costello AC, Kim JH, Johnson GV (1995) Metal-catalyzed oxidation of bovine neurofilaments in vitro. Free Radic Biol Med 18:891–899
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med 32:1050–1060
Andrus PK, Fleck TJ, Gurney ME, Hall ED (1998) Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 71:2041–2048
Smerjac S, Bizzozero OA (2006) Increased protein carbonylation in experimental allergic encephalomyelitis. J Neurochem 96(Suppl 1):PTW07–7
Acknowledgements
This work was supported by PHHS grants NS 47448 and IMSD GM 60201 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue in honor of Naren Banik.
Dr. Oscar Bizzozero dedicates this article to Dr. Naren Banik, outstanding scientist and colleague, in honor to his life-long achievements in the area of spinal cord injury and multiple sclerosis.
Rights and permissions
About this article
Cite this article
Bizzozero, O.A., Reyes, S., Ziegler, J. et al. Lipid Peroxidation Scavengers Prevent the Carbonylation of Cytoskeletal Brain Proteins Induced by Glutathione Depletion. Neurochem Res 32, 2114–2122 (2007). https://doi.org/10.1007/s11064-007-9377-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9377-y