Abstract
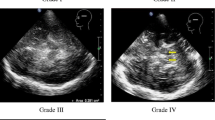
A number of investigations have provided evidence for a central role of iron in the pathogenesis of Parkinson’s disease (PD). Recently it could be demonstrated that iron related changes of the substantia nigra may be one important factor contributing to the hyperechogenicity typicall visualized by transcranial sonography in idiopathic PD. Moreover, also patients with monogenetically caused PD show this hyperechogenicity, although to a lesser extent. According to numerous findings and experiments it seems plausible that iron also contributes to the pathophysiological cascades in the monogenetic forms of PD. Therefore, it is not only essential to acknowledge the pivotal role of iron for PD, but also to enhance the effort in finding therapeutic strategies to prevent the impact of iron on neurodegenerative processes. Moreover, early detection of subjects at risk is essential for the application of therapeutic strategies at a stage at which neuroprotection is still possible.

Similar content being viewed by others
References
Youdim MB, Green AR (1978) Iron deficiency and neurotransmitter synthesis and function. Proc Nutr Soc 37:173–179
Youdim MB (1988) Iron in the brain: implications for Parkinson’s and Alzheimer’s diseases. Mt Sinai J Med 55:97–101
Youdim MB, Ben-Shachar D, Riederer P (1991) Iron in brain function and dysfunction with emphasis on Parkinson’s disease. Eur Neurol 31(Suppl 1):34–40
Youdim MB, Ben-Shachar D, Riederer P (1993) The possible role of iron in the etiopathology of Parkinson’s disease. Mov Disord 8:1–12
Youdim MB, Ben-Shachar D (1987) Minimal brain damage induced by early iron deficiency: modified dopaminergic neurotransmission. Isr J Med Sci 23:19–25
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Riederer P, Rausch WD, Schmidt B, Kruzik P, Konradi C, Sofic E, Danielczyk W, Fischer M, Ogris E (1988) Biochemical fundamentals of Parkinson’s disease. Mt Sinai J Med 55:21–28
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB (1988) Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74:199–205
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52:515–520
Dexter DT, Sian J, Jenner P, Marsden CD (1993) Implications of alterations in trace element levels in brain in Parkinson’s disease and other neurological disorders affecting the basal ganglia. Adv Neurol 60:273–281
Gerlach M, Ben-Shachar D, Riederer P, Youdim MB (1994) Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem 63:793–807
Youdim MB, Ben-Shachar D, Riederer P (1989) Is Parkinson’s disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol Scand Suppl 126:47–54
Riederer P, Youdim MBH (eds) (1993) Iron in central nervous system disorders. Springer, Vienna
Gotz ME, Double K, Gerlach M, Youdim MB, Riederer P (2004) The relevance of iron in the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci 1012:193–208
Bartzokis G, Cummings J, Perlman S, Hance DB, Mintz J (1999) Increased basal ganglia iron levels in Huntington disease. Arch Neurol 56:569–574
Hutchinson M, Raff U (2000) Structural changes of the substantia nigra in Parkinson’s disease as revealed by MR imaging. AJNR Am J Neuroradiol 21:697–701
Mondino F, Filippi P, Magliola U, Duca S (2002) Magnetic resonance relaxometry in Parkinson’s disease. Neurol Sci 23(Suppl 2):S87–S88
Brass SD, Chen NK, Mulkern RV, Bakshi R (2006) Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging 17:31–40
Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P (2007) Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov Disord 22:334–340
Berg D, Behnke S, Walter U (2006) Application of transcranial sonography in extrapyramidal disorders: updated recommendations. Ultraschall Med 27:12–19
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184
Berg D, Siefker C, Becker G (2001) Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 248:684–689
Walter U, Wittstock M, Benecke R, Dressler D (2002) Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. J Neural Transm 109:191–196
Sommer U, Hummel T, Cormann K, Mueller A, Frasnelli J, Kropp J, Reichmann H (2004) Detection of presymptomatic Parkinson’s disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord 19:1196–1202
Miwa H, Okawa M, Kondo T (2006) [Echogenicity of the substantia nigra in Parkinson’s disease: its relation to clinical findings]. No To Shinkei 58:199–204
Spiegel J, Hellwig D, Mollers MO, Behnke S, Jost W, Fassbender K, Samnick S, Dillmann U, Becker G, Kirsch CM (2006) Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson’s disease. Brain 129:1188–1193
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60:74–77
Behnke S, Berg D, Naumann M, Becker G (2005) Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry 76:423–425
Berg D, Grote C, Rausch WD, Maurer M, Wesemann W, Riederer P, Becker G (1999) Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol 25:901–904
Berg D, Becker G, Zeiler B, Tucha O, Hofmann E, Preier M, Benz P, Jost W, Reiners K, Lange KW (1999) Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology 53:1026–1031
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P, Tucha O, Preier M, Lange KW, Reiners K, Gerlach M, Becker G (2002) Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 59:999–1005
Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, Tampellini D, Riederer P, Gerlach M, Becker G (2005) In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord 20:1278–1285
Gandon Y, Guyader D, Heautot JF, Reda MI, Yaouanq J, Buhe T, Brissot P, Carsin M, Deugnier Y (1994) Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging. Radiology 193:533–538
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20:383–385
Brooks DJ (1998) The early diagnosis of Parkinson’s disease. Ann Neurol 44:S10–S18
Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P (2004) PET and SPECT functional imaging studies in Parkinsonian syndromes: from the lesion to its consequences. Neuroimage 23:1–16
Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G (2001) Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry 50:463–467
Berg D, Siefker C, Ruprecht-Dorfler P, Becker G (2001) Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology 56:13–17
de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alperovitch A, Rocca WA (1997) Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:10–15
Schweitzer KJ, Hilker R, Walter U, Burghaus L, Berg D (2006) Substantia nigra hyperechogenicity as a marker of predisposition and slower progression in Parkinson’s disease. Mov Disord 21:94–98
Ruprecht-Dorfler P, Berg D, Tucha O, Benz P, Meier-Meitinger M, Alders GL, Lange KW, Becker G (2003) Echogenicity of the substantia nigra in relatives of patients with sporadic Parkinson’s disease. Neuroimage 18:416–422
Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Durr A, Grandchamp B (2002) Association study between iron-related genes polymorphisms and Parkinson’s disease. J Neurol 249:801–804
Hochstrasser H, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, Zeiler B, Bornemann A, Pahnke J, Becker G, Riess O, Berg D (2004) Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson disease. Neurology 63:1912–1917
Felletschin B, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, Sommer U, Zeiler B, Becker G, Riess O, Berg D (2003) Screening for mutations of the ferritin light and heavy genes in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. Neurosci Lett 352:53–56
Deplazes J, Schobel K, Hochstrasser H, Bauer P, Walter U, Behnke S, Spiegel J, Becker G, Riess O, Berg D (2004) Screening for mutations of the IRP2 gene in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. J Neural Transm 111:515–521
Akbas N, Hochstrasser H, Deplazes J, Tomiuk J, Bauer P, Walter U, Behnke S, Riess O, Berg D (2006) Screening for mutations of the HFE gene in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. Neurosci Lett 407:16–19
Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G (2001) Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem 79:225–236
Klomp LW, Gitlin JD (1996) Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet 5:1989–1996
Kohno S, Miyajima H, Takahashi Y, Inoue Y (2000) Aceruloplasminemia with a novel mutation associated with parkinsonism. Neurogenetics 2:237–238
Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, Burn J (2001) Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 28:350–354
Bosio S, De Gobbi M, Roetto A, Zecchina G, Leonardo E, Rizzetto M, Lucetti C, Petrozzi L, Bonuccelli U, Camaschella C (2002) Anemia and iron overload due to compound heterozygosity for novel ceruloplasmin mutations. Blood 100:2246–2248
Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J, Connor J (2003) Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 71:46–63
LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, Switzer R III, Grinberg A, Love P, Tresser N, Rouault TA (2001) Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27:209–214
Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MB (2005) Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann N Y Acad Sci 1053:356–375
Gasser T (2005) Genetics of Parkinson’s disease. Curr Opin Neurol 18:363–369
Klein UC, Hilker R, Benecke R, Pramstaller PP, Dressler D (2004) Brain parenchyma sonography detects preclinical parkinsonism. Mov Disord 19:1445–1449
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173
Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B (2002) Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J Biol Chem 277:16116–16123
Hashimoto M, Rockenstein E, Crews L, Masliah E (2003) Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med 4:21–36
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–989
Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P (2000) Crosslinking of alpha-synuclein by advanced glycation endproducts—an early pathophysiological step in Lewy body formation? J Chem Neuroanat 20:253–257
Lee EN, Lee SY, Lee D, Kim J, Paik SR (2003) Lipid interaction of alpha-synuclein during the metal-catalyzed oxidation in the presence of Cu2+ and H2O2. J Neurochem 84:1128–1142
Stadtman ER (2001) Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci 928:22–38
Cole NB, Murphy DD, Lebowitz J, Di Noto L, Levine RL, Nussbaum RL (2005) Metal-catalyzed oxidation of alpha-synuclein: helping to define the relationship between oligomers, protofibrils, and filaments. J Biol Chem 280:9678–9690
Jenner P, Olanow CW (1998) Understanding cell death in Parkinson’s disease. Ann Neurol 44:S72–S84
Gaeta A, Hider RC (2005) The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol 146:1041–1059
Castellani RJ, Siedlak SL, Perry G, Smith MA (2000) Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol (Berl) 100:111–114
Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D (2001) alpha-Synuclein implicated in Parkinson’s disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med 30:1163–1170
Junn E, Mouradian MM (2002) Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett 320:146–150
Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR (2002) Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36:1007–1019
Tsai YC, Fishman PS, Thakor NV, Oyler GA (2003) Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J Biol Chem 278:22044–22055
Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A (2003) Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet 12:517–526
Jiang H, Ren Y, Zhao J, Feng J (2004) Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet 13:1745–1754
Kann M, Jacobs H, Mohrmann K, Schumacher K, Hedrich K, Garrels J, Wiegers K, Schwinger E, Pramstaller PP, Breakefield XO, Ozelius LJ, Vieregge P, Klein C (2002) Role of parkin mutations in 111 community-based patients with early-onset parkinsonism. Ann Neurol 51:621–625
Foroud T, Uniacke SK, Liu L, Pankratz N, Rudolph A, Halter C, Shults C, Marder K, Conneally PM, Nichols WC (2003) Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology 60:796–801
Mata IF, Lockhart PJ, Farrer MJ (2004) Parkin genetics: one model for Parkinson’s disease. Hum Mol Genet 13(Spec No 1):R127–133
Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, Troncoso J, Dawson VL, Dawson TM, Lim KL (2005) Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum Mol Genet 14:3885–3897
Jimenez Del Rio M, Moreno S, Garcia-Ospina G, Buritica O, Uribe CS, Lopera F, Velez-Pardo C (2004) Autosomal recessive juvenile parkinsonism Cys212Tyr mutation in parkin renders lymphocytes susceptible to dopamine- and iron-mediated apoptosis. Mov Disord 19:324–330
Pawlyk AC, Giasson BI, Sampathu DM, Perez FA, Lim KL, Dawson VL, Dawson TM, Palmiter RD, Trojanowski JQ, Lee VM (2003) Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem 278:48120–48128
Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science 304:1328–1331
Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA (2004) Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA 101:10810–10814
Lipton SA, Nakamura T, Yao D, Shi ZQ, Uehara T, Gu Z (2005) Comment on “S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function”. Science 308:1870; author reply 1870
Lim KL, Dawson VL, Dawson TM (2002) The genetics of Parkinson’s disease. Curr Neurol Neurosci Rep 2:439–446
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–S36; discussion S36–S28
Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR Jr, Gong J, Abasi H, Blumberg J, Taylor A (1997) Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem 272:28218–28226
Nishikawa K, Li H, Kawamura R, Osaka H, Wang YL, Hara Y, Hirokawa T, Manago Y, Amano T, Noda M, Aoki S, Wada K (2003) Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochem Biophys Res Commun 304:176–183
Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L (2004) Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem 279:13256–13264
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40:427–446
Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL (2006) The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol 356:1036–1048
Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A (2004) DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol 2:e362
Schapira AH (2006) Etiology of Parkinson’s disease. Neurology 66:S10–S23
Ben-Shachar D, Eshel G, Finberg JP, Youdim MB (1991) The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem 56:1441–1444
Youdim MB, Stephenson G, Ben Shachar D (2004) Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann N Y Acad Sci 1012:306–325
Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MB (2004) Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology 46:254–263
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Moussa Youdim
Rights and permissions
About this article
Cite this article
Berg, D. Disturbance of Iron Metabolism as a Contributing Factor to SN Hyperechogenicity in Parkinson’s Disease: Implications for Idiopathic and Monogenetic Forms. Neurochem Res 32, 1646–1654 (2007). https://doi.org/10.1007/s11064-007-9346-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9346-5