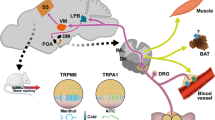
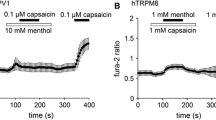
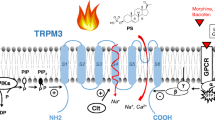
Transient receptor potential melastatin 8 channels (TRPM8s) are molecular sensors of temperature. They are expressed in primary afferent neurons (PANs) and function as receptors of innocuous cold. Depolarization of sensory endings of PANs by cooling-induced TRPM8 current is transduced into patterns of action potentials, encoding information about temperature and conveying it to the CNS, where an adequate thermoregulatory central response is generated. Under certain conditions, the receptor coding may appear misleading; e.g., selective activation of TRPM8 by agonists (menthol, icilin, or eucalyptol) elicits a coolness sensation in the absence of actual cooling. Both binding of such “cool mimetics” and physical cooling exert a similar effect on TRPM8s; the factor induces a hyperpolarization shift and generation of spiking patterns reporting about cooling of the environment and, thus, conveying false information in the former case. Application of volatile anesthetics (VAs) was recently shown to produce a similar shift of TRPM8 activation. This effect was assumed to underlie the adverse effects of VAs, such as shivering and a cooling sensation at the beginning of anesthesia, even at warm temperatures in the surgery theater. To validate this assumption, the VA effects on the activity patterns of the cool receptor should be investigated. Here, we upgraded an established model of the thermoreceptor by incorporating a prototype-based mechanism of VA-induced TRPM8 activation. On this model, we compared the activity patterns generated by the receptor at real cooling (VA–) and the VA-induced (“false”) activation of TRPM8 (VA+). We demonstrated that, when affected by VA at a neutral (warm) temperature, the cold receptor activity switches from tonic singlet spiking to doublet bursting. We demonstrated that, under both conditions, VA– and VA+, along with cooling the receptor model exhibits a sequence of transitions between different patterns of impulse activities, single-spike, doublet, triplet, and quadruplet spiking. The VA+ receptor model is specifically characterized by starting these transitions earlier and completing the sequence in a narrower range and at a higher temperature. The average spiking rate of the VA+ receptor model was notably higher than that in the VA– one, and bursting patterns included greater numbers of spikes for any given coded temperature.
Similar content being viewed by others
References
K. Nakamura, “Central circuitries for body temperature regulation and fever,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 301, No. 5, 1207–1228 (2011).
K. Nakamura, “Afferent pathways for autonomic and shivering thermoeffectors,” Handb. Clin. Neurol., 156, 263–279 (2018).
K. Uchida, K. Dezaki, T. Yoneshiro, et al., “Involvement of thermosensitive TRP channels in energy metabolism,” J. Physiol. Sci., 67, No. 5, 549–560 (2017).
T. Yahiro, N. Kataoka, Y. Nakamura, et al., “The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioral thermoregulation,” Sci. Rep., 7, No. 1, 5031 (2017).
K. Tajino, K. Matsumura, K. Kosada, et al., “Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 293, No. 5, 2128–2135 (2007).
D. I. Sessler, “Perioperative thermoregulation and heat balance,” Lancet, 387, No. 10038, 2655–2664 (2016).
D. D. McKemy, W. M. Neuhausser, and D. Julius, “Identification of a cold receptor reveals a general role for TRP channels in thermosensation,” Nature, 416, No. 6876, 52–58 (2002).
A. M. Peier, A. Moqrich, A. C. Hergarden, et al., “A TRP channel that senses cold stimuli and menthol,” Cell, 108, No. 5, 705–715 (2002).
N. J. Himmel, J. M. Letcher, A. Sakurai, et al., “Drosophila menthol sensitivity and the Precambrian origins of transient receptor potential-dependent chemosensation,” Philos. Trans. R. Soc. Lond. B Biol. Sci., 374, No. 1785, 20130369 (2019).
N. J. Himmel and D. N. Cox, “Transient receptor potential channels: current perspectives on evolution, structure, function and nomenclature,” Proc. Biol. Sci., 287, No. 1933, 1–9 (2020).
N. Raddatz, J. P. Castillo, C. Gonzalez, et al., “Temperature and voltage coupling to channel opening in transient receptor potential melastatin 8 (TRPM8),” J. Biol. Chem., 289, No. 51, 35438–35454 (2014).
T. Quallo, N. Vastani, E. Horridge, et al., “TRPM8 is a neuronal osmosensor that regulates eye blinking in mice,” Nat. Commun., 6, 7150 (2015).
D. A. Andersson, H. W. Chase, and S. Bevan, “TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH,” J. Neurosci., 24, No. 23, 5364–5369 (2004).
Y. Yin, M. Wu, L. Zubcevic, et al., “Structure of the coldand menthol-sensing ion channel TRPM8,” Science, 359, No. 6372, 237–241 (2018).
M. Iftinca and C. Altier, “The cool things to know about TRPM8!,” Channels (Austin), 14, No. 1, 413–420 (2020).
M. M. Diver, Y. Cheng, and D. Julius, “Structural insights into TRPM8 inhibition and desensitization,” Science, 365, No. 6460, 1434–1440 (2019).
D. M. Bautista, J. Siemens, J. M. Glazer, et al., “The menthol receptor TRPM8 is the principal detector of environmental cold,” Nature, 448, No. 7150, 204–208 (2007).
A. Dhaka, A. N. Murray, J. Mathur, et al., “TRPM8 is required for cold sensation in mice,” Neuron, 54, No. 3, 371–378 (2007).
R. W. Colburn, M. L. Lubin, D. J. Stone Jr., et al., “Attenuated cold sensitivity in TRPM8 null mice,” Neuron, 54, No. 3, 379–386 (2007).
K. Tajino, H. Hosokawa, S. Maegawa, et al., “Coolingsensitive TRPM8 is thermostat of skin temperature against cooling,” PLoS One, 6, No. 3, e17504 (2011).
M. C. Almeida, T. Hew-Butler, R. N. Soriano, et al., “Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature,” J. Neurosci., 32, No. 6, 2086–2099 (2012).
T. Voets, G. Droogmans, U. Wissenbach, et al., “The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels,” Nature, 430, No. 7001, 748–754 (2004).
F. Vanden Abeele, A. Kondratskyi, C. Dubois, et al., “Complex modulation of the cold receptor TRPM8 by volatile anesthetics and its role in complications of general anesthesia,” J. Cell. Sci., 126, Pt. 19, 4479–4489 (2013).
E. Olivares, S. Salgado, J. P. Maidana, et al., “TRPM8-dependent dynamic response in a mathematical model of cold thermoreceptor,” PLoS One, 10, No. 10, e0139314 (2015).
N. T. Carnevale and M. L. Hines, The NEURON Book, Cambridge Univ. Press, Cambridge (2006).
H. A. Braun, M. T. Huber, M. Dewald, et al., “Computer simulations of neuronal signal transduction: the role of nonlinear dynamics and noise,” Int. J. Bifurc. Chaos, 8, No. 5, 881–889 (1998).
H. A. Braun, H. Bade, and H. Hensel, “Static and dynamic discharge patterns of bursting cold fibers related to hypothetical receptor mechanisms,” Pflügers Arch., 386, No. 1, 1–9 (1980).
H. A. Braun, M. T. Huber, N. Anthes, et al., “Interactions between slow and fast conductances in the Huber/Barun model of cold-receptor discharges,” Neurocomputing, 32–33, 51–59 (2000).
S. M. Korogod and L. E. Demianenko, “Temperature effects on non-TRP ion channels and neuronal excit-ability,” Opera Med. Physiol., 3, 84–92 (2017).
K. F. Herold and H. C. Jr. Hemmings, “Sodium channels as targets for volatile anesthetics,” Front. Pharmacol., 3, 50 (2012).
S. Rajagopal, S. R. Sangam, and S. Singh, “Differential regulation of volatile anesthetics on ion channels,” Int. J. Nutr. Pharmacol. Neurol. Dis., 5, No. 4, 128–134 (2015).
N. Denomme, J. M. Hull, and G. A. Mashour, “Role of voltage-gated sodium channels in the mechanism of ether-induced unconsciousness,” Pharmacol Rev., 71, No. 4, 450–466 (2019).
A. Kurz, J. Xiong, D. I. Sessler, et al., “Isoflurane produces marked and nonlinear decreases in the vasoconstriction and shivering thresholds,” Ann. N.Y. Acad. Sci., 813, 778–785 (1997).
W. Xia, H. Fu, H. Liu, et al., “A test-retest reliability study of assessing small cutaneous fibers by measuring current perception threshold with pin electrodes,” PLoS One, 15, No. 11, e0242490 (2020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korogod, S.M., Maksymchuk, N.V., Demianenko, L.E. et al. Adverse Modulation of the Firing Patterns of Cold Receptors by Volatile Anesthetics Affecting Activation of TRPM8 Channels: a Modeling Study. Neurophysiology 52, 324–333 (2020). https://doi.org/10.1007/s11062-021-09889-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-021-09889-2