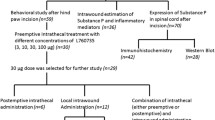
We investigated the effectiveness of intrathecal introduction of GABAA and GABAB receptor agonists in reversing pain modalities in a central model of neuropathic pain. In adult male Wistar rats, spinal cord injury was produced by compression of the spinal cord at the T6–T8 level. For drug delivery to the spinal cord, a PE10 catheter was inserted into the subarachnoid space through an intervertebral foramen. Testing was performed three weeks after spinal cord injury. Mechanical and thermal hyperalgesia were estimated using the pressure withdrawal (Randall–Selitto) and heat withdrawal (Hargreaves) tests, respectively. Tactile allodynia was quantitated by testing with the von Frey filaments. Cold allodynia was assessed by the acetone drop test to the hindpaw. The motor function was evaluated using the 21-score scale for locomotion in the open field. At the end of the experiment, animals were anesthetized and transcardially perfused with fixative, and sections of the spinal cord were prepared and stained. Compression injury of the spinal cord produced a cavity in the dorsal horn of the spinal cord and resulted in clear impairment of the motor function of animals. Spinal administration of both abovementioned drugs reduced mechanical hyperalgesia and cold and tactile allodynia. The GABAA agonist muscimol also decreased thermal hyperalgesia, while the GABAB agonist baclofen did not change this phenomenon. Thus, exogenous administration of GABAA agonists can reverse all spinal cord compression-induced sensory disorders. These agents may provide a better therapy of neuropathic pain than GABAB agonists.
Similar content being viewed by others
References
H. Ueda, H. Matsunaga, O. I. Olaposi, and J. Nagai, “Lysophosphatidic acid: chemical signature of neuropathic pain,” Biochim. Biophys. Acta, 1831, No. 1, 61–73 (2013).
M. Hosseini, Z. Karami, A. Janzadenh, et al., “The effect of intrathecal administration of muscimol on modulation of neuropathic pain symptoms resulting from spinal cord injury; an experimental study,” Emergency, 2, No. 4, 151–157 (2014).
H. U. Zeilhofer, D. Benke, and G. E. Yevenes, “Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control,” Annu. Rev. Pharmacol. Toxicol., 52, 111–133 (2012).
J. M. Fuks, R. B. Arrighi, J. M. Weidner, et al., “GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii,” PLoS Pathog., 8, No. 12, e1003051 (2012).
P. J. Kammermeier and J. Yun, “Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits,” J. Pharmacol. Exp. Ther., 312, No. 2, 502–508 (2005).
G. Munro, R. R. Hansen, and N. R. Mirza, “GABA(A) receptor modulation: potential to deliver novel pain medicines?” Eur. J. Pharmacol., 716, Nos. 1–3, 17–23 (2013).
S. J. Enna and K. E. McCarson, “The role of GABA in the mediation and perception of pain,” Adv. Pharmacol., 54, 1–27 (2006).
A. A. van der Plas, M. A. van Rijn, J. Marinus, et al., “Efficacy of intrathecal baclofen on different pain qualities in complex regional pain syndrome,” Anesth. Analg., 116, No. 1, 211–215 (2013).
A. A. van der Plas, J. Marinus, S. Eldabe, et al., “The lack of efficacy of different infusion rates of intrathecal baclofen in complex regional pain syndrome: a randomized, double-blind, crossover study,” Pain Med., 12, No. 3, 459–465 (2011).
T. Ishikawa, M. Marsala, T. Sakabe, and T. L. Yaksh, “Characterization of spinal amino acid release and touch-evoked allodynia produced by spinal glycine or GABA(A) receptor antagonist,” Neuroscience, 95, No. 3, 781–786 (2000).
K. Kataoka, K. Hara, Y. Haranishi, et al., “The antinociceptive effect of SNAP5114, a gamma-aminobutyric acid transporter-3 inhibitor, in rat experimental pain models,” Anesth. Analg., 116, No. 5, 1162–1169 (2013).
D. Bian, M. H. Ossipov, C. Zhong, et al., “Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury,” Neurosci. Lett., 241, Nos. 2–3, 79–82 (1998).
H. Sun, K. Ren, C. M. Zhong, et al., “Nerve injuryinduced tactile allodynia is mediated via ascending spinal dorsal column projections,” Pain, 90, Nos. 1–2, 105–111 (2001).
J. H. Hwang and T. L. Yaksh, “The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat,” Pain, 70, No. 1, 15–22 (1997).
G. Schechtmann, G. Lind, J. Winter, et al., “Intrathecal clonidine and baclofen enhance the pain-relieving effect of spinal cord stimulation: a comparative placebocontrolled, randomized trial,” Neurosurgery, 67, No. 1, 173–181 (2010).
S. Tasseel Ponche, A. L. Ferrapie, A. Chenet, et al., “Intrathecal baclofen in cerebral palsy. A retrospective study of 25 wheelchair-assisted adults,” Ann. Phys. Rehabil.. Med., 53, No. 8, 483–498 (2010).
Medical Advisory Secretariat, “Intrathecal baclofen pump for spasticity: an evidence-based analysis,” Ont. Health Technol. Assess Ser., 5, No. 7, 1–93 (2005).
F. Xu, T. Li, and B. Zhang, “An improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of rats,” J. Neurosci. Methods, 183, No. 2, 114–118 (2009).
D. M. Basso, M. S. Beattie, and J. C. Bresnahan, “Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection,” Exp. Neurol., 139, No. 2, 244–256 (1996).
J. Lei and H. J. You, “Endogenous descending facilitation and inhibition differ in control of formalin intramuscularly induced persistent muscle nociception,” Exp. Neurol., 248, 100–111 (2013).
K. Fukuhara, T. Katafuchi, and M. Yoshimura, “Effects of baclofen on mechanical noxious and innocuous transmission in the spinal dorsal horn of the adult rat: in vivo patch-clamp analysis,” Eur. J. Neurosci., 38, No. 10, 3398–3407 (2013).
J. Paul, H. U. Zeilhofer, and J. M. Fritschy, “Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents,” J. Comp. Neurol., 520, No. 17, 3895–3911 (2012).
J. M. Castro-Lopes, M. Malcangio, B. H. Pan, and N. G. Bowery, “Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy,” Brain Res., 679, No. 2, 289–297 (1995).
T. L. Yaksh, “Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists,” Pain, 37, No. 1, 111–123 (1989).
J. X. Hao, X. J. Xu, and Z.Wiesenfeld-Hallin, “Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat,” Neurosci. Lett., 182, No. 2, 299–302 (1994).
F. Viguier, B. Michot, V. Kayser, et al., “GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain,” Neuropharmacology, 63, No. 6, 1093–1106 (2012).
E. Redondo-Castro, G. Garcia-Alias, and X. Navarro, “Plastic changes in lumbar segments after thoracic spinal cord injuries in adult rats: an integrative view of spinal nociceptive dysfunctions,” Restor. Neurol. Neurosci., 31, No. 4, 411–430 (2013).
T. P. Malan, H. P. Mata, and F. Porreca, “Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain,” Anesthesiology, 96, No. 5, 1161–1167 (2002).
D. Zurowski, L. Nowak, A. Machowska, et al., “Exogenous melatonin abolishes mechanical allodynia but not thermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine-gabaergic mechanism,” J. Physiol. Pharmacol., 63, No. 6, 641–647 (2012).
M. H. Ossipov, J. Lai, T. P. Malan, Jr, and F. Porreca, “Spinal and supraspinal mechanisms of neuropathic pain,” Ann. N.Y. Acad. Sci., 909, 12–24 (2000).
J. X. Hao, I. S. Xu, X. J. Xu, and Z. Wiesenfeld-Hallin, “Effects of intrathecal morphine, clonidine and baclofen on allodynia after partial sciatic nerve injury in the rat,” Acta Anaesthesiol. Scand., 43, No. 10, 1027–1034 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasirinezhad, F., Hosseini, M., Karami, Z. et al. Comparative Efficacy of GABAA and GABAB Receptor Agonists in Pain Alleviation in a Spinal Cord Injury Model of Neuropathic Pain. Neurophysiology 51, 322–331 (2019). https://doi.org/10.1007/s11062-020-09826-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-020-09826-9