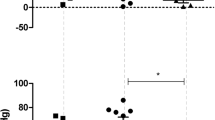
In rats anesthetized with urethane (1.7 g/kg, i. p.), we investigated the effects of stereotaxic microinjections of baclofen into the medullary nuclei involved in the neural control of cardiovascular activity (nuclei paramedianus, ambiguus, and reticularis lateralis). Changes in the hemodynamic parameters (systolic and diastolic blood pressure and heart rate) were measured. Injections of the above GABAB receptor agonist (10–7, 10–6, or 10–5 M, 0.1 μl) into the medullary cardiovascular nuclei was accompanied by changes in the blood pressure, the magnitude and direction of which depended not only on the baclofen concentration but also on the site of injection (into one nucleus or another). Injections of the agent into the nucl. ambiguus at a 10–7 M concentration resulted in an increase in the blood pressure, but a 10–5 M concentration provided significant reduction of the systolic and diastolic blood pressure. If baclofen was injected into the nucl. reticularis lat., the blood pressure also increased or decreased but the concentration dependence was opposite to the above described. Injections of the agent into the nucl. paramedianus were always accompanied by significant increases in the blood pressure. Changes in the heart rate following baclofen injections into the nuclei under study were insignificant. The specificities of the baclofen-induced effects are probably related to peculiarities of the functioning of GABAB receptors, the activation of which may mediate the effects of multiple neuronal mechanisms.
Similar content being viewed by others
References
S. See and R. Ginzburg, “Skeletal muscle relaxants, ” Pharmacotherapy, 2, No. 28, 207-213 (2008)
M. Brusberg, A. Ravnefjord, R. Martinsson, et al., “The GABA(B) receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats, ”Neuropharmacology, 56, No. 2, 362-367 (2009).
A. F. Sved and Ju. C. Sved, “Endogenous GABA acts on GABAB receptors in nucleus tractus solitarius to increase blood pressure,” Brain Res., 526, No. 2, 235-240 (1990).
A. Florentino, K. Varga, and G.Kunos, “Mechanism of the cardiovascular effects of GABAB receptor activation in the nucleus tractus solitarii of rats,” Brain Res., 535, 264–270 (1990).
P. A. Brooks, S. R. Glaum, R. J. Miller, and K. M. Spyer, “The actions of baclofen on neurones and synaptic transmission in the nucleus tractus solitarii of the rat in vitro,” J. Physiol., 457, 115–129 (1992).
J. C. Callera, L. G. Bonagamba, A. Nosjean, et al., “Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia,” Auton. Neurosci., 84 , 58–67 (2000).
W. Zhang, M. Herrera-Rosales, and S.Mifflin, “Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius,” Hypertension, 49, 659-663 (2007).
W. Zhang and S. Mifflin, “Chronic hypertension enhances presynaptic inhibition by baclofen in the nucleus of the solitary tract,” Hypertension, 55, 481-486 (2010).
.B. Li., Liu Qing, X. Chengluan, et al., “GABAB receptor gene transfer into the nucleus tractus solitarii induces chronic blood pressure elevation in normotensive rats,” Circulation, 77, No. 10, 2558–2566 (2013).
T. S. Moreira, A. C. Takakura, and E. Colombari, “Important GABAergic mechanism within the NTS and the control of sympathetic baroreflex in SHR,” Autonom. Neurosci., 159, 62–70 (2011).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, New York, (1982).
N. V. Radchenko, L. N. Shapoval, T. L. Davydovskaya, et al., “Features of GABAergic cardiovascular control provided by medullary neurons in rats,” Neurophysiology, 45, No. 5, 407-417 (2013).
L. A. Chahl and S. B. Walker, “The effect of baclofen on the cardiovascular system of the rat,” Br. J. Pharmacol., 69, 631–637 (1980).
Y. Takemoto, “Hindquarters vasoconstriction through central GABAB receptors in conscious rats,” Exp. Physiol., 88, No. 4, 491-497 (2003).
K. Hayakawa, M. Kimura, and K. Kamata, “Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats,” Eur. J. Pharmacol., 438, No. 1-2,107-113 (2002).
F. Yao, C. Summers, S. T. O’Rourke, and C. Sun, “Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats,” Am. J. Physiol. Heart Circ. Physiol, 294, H2712-H2720 (2008).
Q. Zhang., F. Yao, S. T. O’Rourke, et al., “Angiotensin II enhances GABAB receptor-mediated responses and expression in nucleus tractus solitarii of rats,” Am. J. Physiol. Heart Circ. Physiol., 297, H1837–H1844 (2009).
C. D. Landulpho, A. C Dias, and E. Colombari, “Cardiovascular mechanisms activated by microinjection of baclofen into NTS of conscious rats,” Am. J. Physiol. Heart Circ. Physiol., 284, H987–H993 (2003).
V. C. Chitravanshi, K. Kawabe, and H. N. Sapru, “GABA and glycine receptors in the nucleus ambiguus mediate tachycardia elicited by chemical stimulation of the hypothalamic arcuate nucleus,” Am. J. Physiol. Heart Circ. Physiol., 309, H174-H184 (2015).
R. J. Bateman, C. R. Boychuk, K. E. Phibin, and D. Mendelowitz, “Beta adrenergic receptor modulation of neurotransmisdsion to cardiac vagal neurons in the nucleus ambiguus,” Neuroscience , 210, 58-66 (2010).
M. Zhang, Y. T. Wang, D. M. Vyas, et al., “Nicotinic cholinoceptor-mediated excitatory postsynaptic potentials in rat nucleus ambiguus,” Exp. Brain Res., 96, 83-88 (1993).
R. Stoop, “Neuromodulation by oxytocin and vasopressin,” Neuron, 76, 142-159 (2012).
R. Becker, I. Benes, U. Sure, et al., “Intrathecal baclofen alleviates autonomic dysfunction in severe brain injury,” J. Clin. Neurosci., 7, 316-319 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapoval, L.M., Dmytrenko, O.V., Naumenko, A.M. et al. Effects of Stereotactic Introduction of Baclofen in the Medullary Cardiovascular Nuclei of Rats. Neurophysiology 49, 30–35 (2017). https://doi.org/10.1007/s11062-017-9626-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-017-9626-x