Abstract
Purpose
Upfront dual checkpoint blockade with immune checkpoint inhibitors (ICI) has demonstrated efficacy for treating melanoma brain metastases (MBM) in asymptomatic patients. Whether the combination of stereotactic radiosurgery (SRS) with dual checkpoint blockade improves outcomes over dual-checkpoint blockade alone is unknown. We evaluated clinical outcomes of patients with MBM receiving ICI with nivolumab and ipilimumab, with and without SRS.
Methods
49 patients with 158 MBM receiving nivolumab and ipilimumab for untreated MBM between 2015 and 2022 were identified at our institution. Patient and tumor characteristics including age, Karnofsky Performance Status (KPS), presence of symptoms, cancer history, MBM burden, and therapy course were recorded. Outcomes measured from initiation of MBM-directed therapy included overall survival (OS), local control (LC), and distant intracranial control (DIC). Time-to-event analysis was conducted with the Kaplan–Meier method.
Results
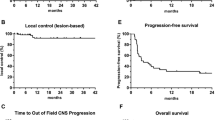
25 patients with 74 MBM received ICI alone, and 24 patients with 84 MBM received concurrent SRS. Median follow-up was 24 months. No differences in age (p = 0.96), KPS (p = 0.85), presence of symptoms (p = 0.79), prior MBM (p = 0.68), prior MBM-directed surgery (p = 0.96) or SRS (p = 0.68), MBM size (p = 0.67), or MBM number (p = 0.94) were seen. There was a higher rate of nivolumab and ipilimumab course completion in the SRS group (54% vs. 24%; p = 0.029). The SRS group received prior immunotherapy more often than the ICI alone group (54% vs. 8.0%; p < 0.001). There was no significant difference in 1-year OS (72% vs. 71%, p = 0.20) and DIC (63% v 51%, p = 0.26) between groups. The SRS group had higher 1-year LC (92% vs. 64%; p = 0.002). On multivariate analysis, LC was improved with combination therapy (AHR 0.38, p = 0.01).
Conclusion
In our analysis, patients who received SRS with nivolumab and ipilimumab had superior LC without increased risk of toxicity or compromised immunotherapy treatment completion despite the SRS cohort having higher rates of prior immunotherapy. Further prospective study of combination nivolumab and ipilimumab with SRS is warranted.


Similar content being viewed by others
Data availability
Summary data supporting the results reported in this article can be requested by emailing the corresponding author.
References
Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A (2021) Epidemiology of melanoma. Med Sci (Basel). https://doi.org/10.3390/medsci9040063
National Cancer Institute (2023) SEER Cancer Stat Facts: Melanoma of the Skin. Accessed July 24. https://seer.cancer.gov/statfacts/html/melan.html
Davies MA, Liu P, McIntyre S et al (2011) Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117(8):1687–1696. https://doi.org/10.1002/cncr.25634
Sloan AE, Nock CJ, Einstein DB (2009) Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 16(3):248–255. https://doi.org/10.1177/107327480901600307
Bates JE, Youn P, Usuki KY et al (2015) Brain metastasis from melanoma: the prognostic value of varying sites of extracranial disease. J Neurooncol 125(2):411–418. https://doi.org/10.1007/s11060-015-1932-9
Agarwala SS, Kirkwood JM, Gore M et al (2004) Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 22(11):2101–2107. https://doi.org/10.1200/jco.2004.11.044
Tallet AV, Azria D, Barlesi F et al (2012) Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol 7(1):77. https://doi.org/10.1186/1748-717X-7-77
Lotze M, Dallal R, Kirkwood J, Flickinger J (2000) Cutaneous Melanoma En: Devita VT, Hellman S, Rosenberg SA, editors. Cancer Principles and Practice of Oncology. Philadelphia: Lippincott-Raven
Bander ED, Yuan M, Carnevale JA et al (2021) Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer 127(12):2062–2073. https://doi.org/10.1002/cncr.33459
Sampson JH, Carter JH Jr, Friedman AH, Seigler HF (1998) Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 88(1):11–20. https://doi.org/10.3171/jns.1998.88.1.0011
Davies MA, Saiag P, Robert C et al (2017) Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 18(7):863–873. https://doi.org/10.1016/s1470-2045(17)30429-1
Margolin K, Ernstoff MS, Hamid O et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13(5):459–465. https://doi.org/10.1016/s1470-2045(12)70090-6
Kluger HM, Chiang V, Mahajan A et al (2019) Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol 37(1):52–60. https://doi.org/10.1200/jco.18.00204
Rulli E, Legramandi L, Salvati L, Mandala M (2019) The impact of targeted therapies and immunotherapy in melanoma brain metastases: a systematic review and meta-analysis. Cancer 125(21):3776–3789. https://doi.org/10.1002/cncr.32375
Tawbi HA, Forsyth PA, Hodi FS et al (2021) Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol 22(12):1692–1704. https://doi.org/10.1016/s1470-2045(21)00545-3
Thompson JF, Williams GJ, Hong AM (2022) Radiation therapy for melanoma brain metastases: a systematic review. Radiol Oncol 56(3):267–284. https://doi.org/10.2478/raon-2022-0032
Galli G, Cavalieri S, Di Guardo L et al (2019) Combination of immunotherapy and brain radiotherapy in metastatic melanoma: a retrospective analysis. Oncol Res Treat 42(4):186–194. https://doi.org/10.1159/000497211
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270–e278. https://doi.org/10.1016/s1470-2045(15)70057-4
Vogelbaum MA, Brown PD, Messersmith H et al (2021) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40(5):492–516. https://doi.org/10.1200/JCO.21.02314
Long GV, Atkinson V, Lo S et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19(5):672–681. https://doi.org/10.1016/S1470-2045(18)30139-6
Tawbi HA, Forsyth PA, Algazi A et al (2018) Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379(8):722–730. https://doi.org/10.1056/NEJMoa1805453
Clair WHS, Given CA (2003) Stereotactic radiosurgery associated neurotoxicity. Technol Cancer Res Treat 2(2):147–151. https://doi.org/10.1177/153303460300200211
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol 12(4):265–282. https://doi.org/10.1016/j.prro.2022.02.003
Patel KR, Shoukat S, Oliver DE et al (2017) Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 40(5):444–450. https://doi.org/10.1097/coc.0000000000000199
Soliman H, Das S, Larson DA, Sahgal A (2016) Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 7(11):12318–12330. https://doi.org/10.18632/oncotarget.7131
Kotecha R, Miller JA, Venur VA et al (2018) Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg 129:50–59. https://doi.org/10.3171/2017.1.Jns162797
Dohm AE, Nakashima JY, Kalagotla H et al (2023) Stereotactic radiosurgery and anti-PD-1 + CTLA-4 therapy, anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitors, or conventional chemotherapy for the management of melanoma brain metastases. Eur J Cancer. https://doi.org/10.1016/j.ejca.2023.113287
Acknowledgements
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JT, MM, JN, AD, and KA. The first draft of the manuscript was written by JT, MM, and KA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Joseph D. Tang: no disclosures. Matthew N. Mills: no disclosures.Justyn Nakashima: no disclosures.Ammoren E. Dohm: no disclosures.Nikhil I. Khushalani: personal consulting fees from Astra-Zeneca, Bristol Myers Squibb, Incyte, Iovance, Merck, Novartis, Nektar, Replimune, Regeneron, Jounce, Castle Biosciences, and Instill Bio; receives research funding from Bristol Myers Squibb, Merck, Novartis, Replimune, Celgene, Regeneron, HUYA Biopharmaceuticals, and GlaxoSmithKline; and has common stock holdings in Bellicum Pharmaceuticals, Amarin Corp, and Asensus Surgical. Peter A. Forsyth: has funding from Pfizer and Celgene and is on the advisory boards of Novocure, BTG, Inovio, AbbVie, Ziopharm, Tocagen, and Pfizer. Michael A. Vogelbaum: has indirect equity and royalty interests in Infuseon Therapeutics, Inc. and has received honoraria from Tocagen, Inc. and Celgene. Evan J. Wuthrick: has served on the advisory board and has received honoraria from Bayer, has received research funding from Bristol-Myers Squibb, and has intellectual property: cGMP as protector for chemotherapy and radiation. Hsiang-Hsuan Michael Yu: has received speaker’s honoraria from BrainLab and is on the advisory boards of Novocure and AbbVie. Daniel E. Oliver: no disclosures. James K.C. Liu: no disclosures. Kamran A. Ahmed: has received research funding from Bristol-Myers Squibb, Eli Lilly, and Genentech.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, J.D., Mills, M.N., Nakashima, J. et al. Clinical outcomes of melanoma brain metastases treated with nivolumab and ipilimumab alone versus nivolumab and ipilimumab with stereotactic radiosurgery. J Neurooncol 166, 431–440 (2024). https://doi.org/10.1007/s11060-023-04543-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04543-9