Abstract
Background
Glioblastoma (GBM) is the most common primary brain tumor in adults. Despite extensive research and clinical trials, median survival post-treatment remains at 15 months. Thus, all opportunities to optimize current treatments and improve patient outcomes should be considered. A recent retrospective clinical study found that taking TMZ in the morning compared to the evening was associated with a 6-month increase in median survival in patients with MGMT-methylated GBM. Here, we hypothesized that TMZ efficacy depends on time-of-day and O6-Methylguanine-DNA Methyltransferase (MGMT) activity in murine and human models of GBM.
Methods and results
In vitro recordings using real-time bioluminescence reporters revealed that GBM cells have intrinsic circadian rhythms in the expression of the core circadian clock genes Bmal1 and Per2, as well as in the DNA repair enzyme, MGMT. Independent measures of MGMT transcript levels and promoter methylation also showed daily rhythms intrinsic to GBM cells. These cells were more susceptible to TMZ when delivered at the daily peak of Bmal1 transcription. We found that in vivo morning administration of TMZ also decreased tumor size and increased body weight compared to evening drug delivery in mice bearing GBM xenografts. Finally, inhibition of MGMT activity with O6-Benzylguanine abrogated the daily rhythm in sensitivity to TMZ in vitro by increasing sensitivity at both the peak and trough of Bmal1 expression.
Conclusion
We conclude that chemotherapy with TMZ can be dramatically enhanced by delivering at the daily maximum of tumor Bmal1 expression and minimum of MGMT activity and that scoring MGMT methylation status requires controlling for time of day of biopsy.
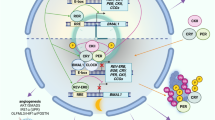
Graphical abstract




Similar content being viewed by others
Data availability
The data generated in this study are available from the corresponding author upon reasonable request.
References
Llaguno SA et al (2009) Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15(1):45–56. https://doi.org/10.1016/j.ccr.2008.12.006
Zong H, Parada LF, Baker SJ (2015) Cell of origin for malignant gliomas and its implication in therapeutic development. Cold Spring Harb Perspect Biol 7(5):a020610. https://doi.org/10.1101/cshperspect.a020610
Tamimi AF, Juweid M (2017) Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications, Brisbane
Cancer of the Brain and Other Nervous System—Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/brain.html. Accessed 24 Mar 2022
Fernandes C et al (2017) Current standards of care in glioblastoma therapy. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications, Brisbane
Hottinger AF, Pacheco P, Stupp R (2016) Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro-Oncol 18(10):1338–1349. https://doi.org/10.1093/neuonc/now182
Hegi ME et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003. https://doi.org/10.1056/NEJMoa043331
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
García M, Clopés A, Bruna J, Martínez M, Fort E, Gil M (2009) Critical appraisal of temozolomide formulations in the treatment of primary brain tumors: patient considerations. Cancer Manag Res 1:137–150
Ortiz R et al (2021) Temozolomide: an updated overview of resistance mechanisms, nanotechnology advances and clinical applications. Curr Neuropharmacol 19(4):513–537. https://doi.org/10.2174/1570159X18666200626204005
Zhang J, Stevens MFG, Bradshaw TD (2012) Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol 5(1):102–114. https://doi.org/10.2174/1874467211205010102
Damato AR et al (2021) Temozolomide chronotherapy in patients with glioblastoma: a retrospective single-institute study. Neuro-Oncol Adv 3(1):vdab041. https://doi.org/10.1093/noajnl/vdab041
Schibler U (2005) The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep 6:S9–S13. https://doi.org/10.1038/sj.embor.7400424
Saini R, Jaskolski M, Davis SJ (2019) Circadian oscillator proteins across the kingdoms of life: structural aspects. BMC Biol 17(1):13. https://doi.org/10.1186/s12915-018-0623-3
Slat EA et al (2017) Cell-intrinsic, Bmal1-dependent circadian regulation of temozolomide sensitivity in glioblastoma. J Biol Rhythms 32(2):121–129. https://doi.org/10.1177/0748730417696788
Dong Z et al (2019) Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov 9(11):1556–1573. https://doi.org/10.1158/2159-8290.CD-19-0215
Trebucq LL, Cardama GA, Menna PL, Golombek DA, Chiesa JJ, Marpegan L (2021) Timing of novel drug 1A–116 to circadian rhythms improves therapeutic effects against glioblastoma. Pharmaceutics 13(7):1091. https://doi.org/10.3390/pharmaceutics13071091
Martineau-Pivoteau N et al (1996) Circadian variation in O6-methylguanine-DNA methyltransferase activity in mouse liver. Anticancer Drugs 7(6):703
Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U (2004) The mammalian circadian timing system: from gene expression to physiology. Chromosoma 113(3):103–112. https://doi.org/10.1007/s00412-004-0296-2
Lévi F, Okyar A, Dulong S, Innominato PF, Clairambault J (2010) Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 50:377–421. https://doi.org/10.1146/annurev.pharmtox.48.113006.094626
Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A (2018) A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173(1):130-139.e10. https://doi.org/10.1016/j.cell.2018.02.017
Uchida Y, Hirayama J, Nishina H (2010) A common origin: signaling similarities in the regulation of the circadian clock and DNA damage responses. Biol Pharm Bull 33(4):535–544. https://doi.org/10.1248/bpb.33.535
Im J-S, Jung B-H, Kim S-E, Lee K-H, Lee J-K (2010) Per3, a circadian gene, is required for Chk2 activation in human cells. FEBS Lett 584(23):4731–4734. https://doi.org/10.1016/j.febslet.2010.11.003
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP (2006) The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22(3):375–382. https://doi.org/10.1016/j.molcel.2006.03.038
Ballesta A, Zhou Q, Zhang X, Lv H, Gallo JM (2014) Multiscale design of cell-type-specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors. CPT Pharmacomet Syst Pharmacol 3(4):e112. https://doi.org/10.1038/psp.2014.9
Rosso L et al (2009) A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res 69(1):120–127. https://doi.org/10.1158/0008-5472.CAN-08-2356
Aasland D, Reich TR, Tomicic MT, Switzeny OJ, Kaina B, Christmann M (2018) Repair gene O6-methylguanine-DNA methyltransferase is controlled by SP1 and up-regulated by glucocorticoids, but not by temozolomide and radiation. J Neurochem. https://doi.org/10.1111/jnc.14262
Kaina B, Christmann M, Naumann S, Roos WP (2007) MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6(8):1079–1099. https://doi.org/10.1016/j.dnarep.2007.03.008
Sarkaria JN et al (2008) Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res Off J Am Assoc Cancer Res 14(10):2900–2908. https://doi.org/10.1158/1078-0432.CCR-07-1719
Switzeny OJ, Christmann M, Renovanz M, Giese A, Sommer C, Kaina B (2016) MGMT promoter methylation determined by HRM in comparison to MSP and pyrosequencing for predicting high-grade glioma response. Clin Epigenet 8(1):49. https://doi.org/10.1186/s13148-016-0204-7
Weller M et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res Off J Am Assoc Cancer Res 21(9):2057–2064. https://doi.org/10.1158/1078-0432.CCR-14-2737
van den Bent MJ et al (2013) MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas a report from EORTC study 26951. Clin Cancer Res Off J Am Assoc Cancer Res 19(19):5513–5522. https://doi.org/10.1158/1078-0432.CCR-13-1157
Yamamuro S et al (2021) Lomustine and nimustine exert efficient antitumor effects against glioblastoma models with acquired temozolomide resistance. Cancer Sci 112(11):4736–4747. https://doi.org/10.1111/cas.15141
Yin A et al (2014) The predictive but not prognostic value of MGMT promoter methylation status in elderly glioblastoma patients: a meta-analysis. PLoS ONE 9(1):e85102. https://doi.org/10.1371/journal.pone.0085102
Hegi ME et al (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol Off J Am Soc Clin Oncol 26(25):4189–4199. https://doi.org/10.1200/JCO.2007.11.5964
Chun LE, Woodruff ER, Morton S, Hinds LR, Spencer RL (2015) Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. J Biol Rhythms 30(5):417–436. https://doi.org/10.1177/0748730415598608
Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM, Champagne J (1993) Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int 10(3):201–204. https://doi.org/10.3109/07420529309073888
Perazzoli G et al (2015) Temozolomide resistance in glioblastoma cell lines: implication of MGMT, MMR, P-glycoprotein and CD133 expression. PLoS ONE 10(10):e0140131. https://doi.org/10.1371/journal.pone.0140131
Giacchetti S et al (2006) Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for research and treatment of cancer chronotherapy group. J Clin Oncol 24(22):3562–3569. https://doi.org/10.1200/JCO.2006.06.1440
Kobayashi M, Wood PA, Hrushesky WJM (2002) Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int 19(1):237–251. https://doi.org/10.1081/cbi-120002600
Damato AR et al (2022) A randomized feasibility study evaluating temozolomide circadian medicine in patients with glioma. Neuro-Oncol Pract 9(3):193–200. https://doi.org/10.1093/nop/npac003
Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA (2008) Redundant function of REV-ERBα and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLOS Genet 4(2):e1000023. https://doi.org/10.1371/journal.pgen.1000023
Zhang EE et al (2009) A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 139(1):199–210. https://doi.org/10.1016/j.cell.2009.08.031
Ramanathan C, Khan SK, Kathale ND, Xu H, Liu AC (2012) Monitoring cell-autonomous circadian clock rhythms of gene expression using luciferase bioluminescence reporters. J Vis Exp 67:e4234
Yoshioka M, Matsutani T, Hara A, Hirono S, Hiwasa T, Takiguchi M, Iwadate Y (2018) Real-time methylation-specific PCR for the evaluation of methylation status of MGMT gene in glioblastoma. Oncotarget 9:27728–27735
Kreth S, Thon N, Eigenbrod S, Lutz J, Ledderose C, Egensperger R, Tonn JC, Kretzschmar HA, Hinske LC, Kreth FW (2011) O-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. PLoS One 6:e17156.
Acknowledgements
We thank the members of the Herzog lab for discussion and comments on the manuscript. This work was supported by National Institutes of Health Grants NINDS R21NS120003 and the Washington University Siteman Cancer Center.
Funding
This work was supported by the National Institutes of Health Grants NINDS R21NS120003 and the Washington University Siteman Cancer Center. Author MFGA was supported by the Washington University Neuroscience Program T32-Training Grant NIH (T32NS121881-01) and the Initiative for Maximizing Student Development (IMSD) Program Training Grant NIH (R25GM103757-10). Author ARD was supported by the National Institutes of Health National Cancer Institute (F31CA250161). Author LLT was supported by grants from Universidad Nacional de Quilmes (PUNQ 2285/22) and by Agencia Nacional de Promoción Científica y Tecnológica de Argentina (PICT 1745-2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Cell experiments and analysis: MFGA, LLT, and TS. Animal experiments and analysis: MFGA, ARD, LLT, and SPCG Mass spectrometry: KC The first draft of the manuscript was written by MFGA, ARD, and EDH. All authors read and approved the final manuscript. MFGA and ARD contributed equally to this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All vertebrate animals in this study were used in accordance with the guidelines established by the Washington University Department of Comparative Medicine (protocol 19-1136, expiration date 05/21/2023, and protocol 23-0105, expiration date 05/17/2026).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez-Aponte, M.F., Damato, A.R., Trebucq, L.L. et al. Circadian regulation of MGMT expression and promoter methylation underlies daily rhythms in TMZ sensitivity in glioblastoma. J Neurooncol 166, 419–430 (2024). https://doi.org/10.1007/s11060-023-04535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04535-9



