Abstract
Purpose
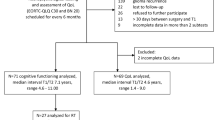
starting from a lack of precise and coherent data in literature, aim of this work is to retrospectively study the influence of chemotherapy with Temozolomide (TMZ) on a wide series of neuropsychological functions in a population of adult high-grade glioma patients.
Methods
an extensive neuropsychological battery was administered pre-operatively (T0) and after 6 (T1) and 12 months (T2) from surgery. After full recovery from surgery, TMZ was delivered concomitant to radiotherapy and, subsequently, adjuvantly for 5-day cycles per month. Parametric and non-parametric analyses were conducted to verify the influence of several aspects of chemotherapy on the adjusted scores of each cognitive test at the two post-operative follow-ups.
Results
Sixty-one patients were included at T0; patients with a lower adjuvant TMZ dosage reported a better performance at the visual attention test at T1, and at the deductive reasoning test at T2. Undergoing more than 8 cycles of adjuvant therapy was slightly associated with a better performance at the long-term verbal memory tasks at T2. No other associations were found with the other cognitive tests and autonomy scales administered.
Conclusions
TMZ proved to be a secure treatment with no negative side effects on cognition and on level of daily autonomy, even at the highest dosage used. This is a positive finding which enables clinicians to reassure patients about the absence of significant negative effects of TMZ on their daily life functioning. In this view, eventual cognitive changes during treatment might not be attributed to chemotherapy but to other events such as tumour relapse.
Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEngl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Scheibel RS, Meyers CA, Levin VA (1996) Cognitive dysfunction following Surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neuro-Oncol 30(1):61–69. https://doi.org/10.1007/BF00177444
Habets EJJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJB (2014) Tumour and Surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir 156(8):1451–1459. https://doi.org/10.1007/s00701-014-2115-8
Zarino B, Di Cristofori A, Fornara GA, Bertani GA et al (2020) Long-term follow-up of neuropsychological functions in patients with high grade gliomas: can cognitive status predict patient’s outcome after Surgery? Acta Neurochir 162:803–812. https://doi.org/10.1007/s00701-020-04230-y
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Durand T, Bernier M-O, Léger I, Taillia H, Noël G, Psimaras D, Ricard D (2015) Cognitive outcome after radiotherapy in Brain Tumor. Curr Opin Oncol 27(6):510–515. https://doi.org/10.1097/CCO.0000000000000227
Kumar G, Dutta P, Parihar VK, Chamallamudi MR, Kumar N (2020) Radiotherapy and its impact on the nervous system of Cancer survivors. CNS Neurol Disord Drug Target 19(5):374–385. https://doi.org/10.2174/1871527319666200708125741
Dubois M, Lapinte N, Villier V, LecointreC, Roy V, Tonon MC et al (2014) Chemotherapy-induced long-term alteration of executive functions and hippocampal cell proliferation: role of glucose as adjuvant. Neuropharmacology 79:234–248. https://doi.org/10.1016/j.neuropharm.2013.11.012
Hermelink K (2015) Chemotherapy and cognitive function in Breast cancer patients: the so-called chemo brain. J Natl Cancer Inst Monogr 2015(51):67–69. https://doi.org/10.1093/jncimonographs/lgv009
Torrente NC, Pastor JB, de la Osa Chaparro N (2020) Systematic review of cognitive sequelae of non-central nervous system cancer and cancer therapy. J Cancer Surviv 1–19. https://doi.org/10.1007/s11764-020-00870-2
Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A (2013) Cognitive effects of chemotherapy in Breast cancer patients: a dose–response study. Psycho-oncology 22:1517–1527. https://doi.org/10.1002/pon.3163
Hsu YH, Chen VCH, Hsieh CC, Weng YP, Hsu YT, Hsiao HPet al et al (2021) Subjective and objective cognitive functioning among patients with Breast cancer: effects of chemotherapy and mood symptoms. Breast Cancer 28:236–245. https://doi.org/10.1007/s12282-020-01168-y
Dwek MR, Rixon L, Hurt C, Simon A et al (2017) Is there a relationship between objectively measured cognitive changes in patients with solid tumours undergoing chemotherapy treatment and their health-related quality of life outcomes? A systematic review. Psycho‐oncology 26(10):1422–1432. https://doi.org/10.1002/pon.4331
Myers JS (2013) Cancer-and chemotherapy-related cognitive changes: the patient experience. In Seminars in oncology nursing.Vol. 29, No. 4, pp. 300–307 https://doi.org/10.1016/j.soncn.2013.08.010
Hodgson KD, Hutchinson AD, Wilson CJ et al (2013) A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev 39(3):297–304. https://doi.org/10.1016/j.ctrv.2012.11.001
Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP (2011) Either called chemobrain or chemofog, the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J Pain Symptom Manag 41(1):126–139. https://doi.org/10.1016/j.jpainsymman.2010.04.021
de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G et al (2012) Late effects of high-dose adjuvant chemotherapy on white and gray matter in Breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp 33(12):2971–2983. https://doi.org/10.1002/hbm.21422
Ponto LLB, Menda Y, Magnotta VA, Yamada TH, Denburg NL, Schultz SK (2015) Frontal hypometabolism in elderly Breast cancer survivors determined by [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. Int J Geriatr Psychiatry 30(6):587–594. https://doi.org/10.1002/gps.4189
Kaiser J, Bledowski C, Dietrich J (2014) Neural correlates of chemotherapy-related cognitive impairment. Cortex 54:33–50. https://doi.org/10.1016/j.cortex.2014.01.010
Xuan H, Gan C, Li W, Huang Z, Wang L, Jia Q et al (2017) Altered network efficiency of functional brain networks in patients with Breast cancer after chemotherapy. Oncotarget 8(62):105648. https://doi.org/10.18632/oncotarget.22358
Cheng H, Li W, Gong L, Xuan H, Huang Z, Zhao H al (2017) Altered resting-state hippocampal functional networks associated with chemotherapy-induced prospective memory impairment in Breast cancer survivors. Sci Rep 7:45135. https://doi.org/10.1038/srep45135
Chiang ACA, Huo X, Kavelaars A, Heijnen CJ (2019) Chemotherapy accelerates age-related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain Behav Immun 79:319–325. https://doi.org/10.1016/j.bbi.2019.04.005
Dietrich J, Prust M, Kaiser J (2015) Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience 309:224–232. https://doi.org/10.1016/j.neuroscience.2015.06.016
Tariq R, Hussain N, Baqai MWS (2023) Factors affecting cognitive functions of patients with high-grade gliomas: a systematic review. Neurol Sci 1–13. https://doi.org/10.1007/s10072-023-06673-4
Mrugala MM, Chamberlain MC (2008) Mechanisms of Disease: temozolomide and glioblastoma–look to the future. Nat Clin Pract Oncol 5(8):476–486. https://doi.org/10.1038/ncponc1155
Pathak N, Cheruku SP, Rao V, Vibhavari RJA, Sumalatha S, Gourishetti K et al (2020) Dehydrozingerone protects temozolomide-induced cognitive impairment in normal and C6 glioma rats besides enhancing its anticancer potential. 3 Biotech 10:1–13. https://doi.org/10.1007/s13205-020-02427-7
Klein M, Drijver AJ, Van Den Bent MJ, Bromberg JC, Hoang-Xuan K, Taphoorn MJ et al (2021) Memory in low-grade glioma patients treated with radiotherapy or temozolomide: a correlative analysis of EORTC study 22033–26033. Neurooncology 23(5):803–811. https://doi.org/10.1093/neuonc/noaa252
Park DY, Tom MC, Chen Y, Tewari S, Ahluwalia MS, Yu JS et al (2022) Cognitive function after concurrent temozolomide-based chemoradiation therapy in low-grade gliomas. J Neurooncol 158(3):341–348. https://doi.org/10.1007/s11060-022-04019-2
Hilverda K, Bosma I, Heimans JJ, Postma TJ, Peter Vandertop W, Slotman BJ, Buter J, Reijneveld JC, Klein M (2010) Cognitive functioning in glioblastoma patients during radiotherapy and temozolomide treatment: initial findings. J Neuro-Oncol 97(1):89–94. https://doi.org/10.1007/s11060-009-9993-2
Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, Fay M, Nishikawa R, Cairncross JG et al (2017) Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. The New England journal of medicine, 376(11), 1027–1037. https://doi.org/10.1056/NEJMoa1611977
Papagno C, Casarotti A, Comi A, Gallucci M, Riva M, Bello L (2012) Measuring clinical outcomes in neuro-oncology. A Battery to evaluate low-grade gliomas (LGG). J Neurooncol 108:269–275. https://doi.org/10.1007/s11060-012-0824-5
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Italian adult population. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 34(5):749–754.Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an https://doi.org/10.1007/s10072-012-1130-x
Carlesimo G, Buccione I, Fadda L et al (2002) Normative data of two memory tasks: short-story Recall and Rey’s figure. Nuova Rivista Di Neurologia, 12(1)
Carlesimo GA, Caltagirone C, Gainotti GUID, Fadda L, Gallassi R, Lorusso S et al (1996) The mental deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36(6):378–384. https://doi.org/10.1159/000117297
Caffarra P, Vezzadini G, Francesca D, Zonato F, Venneri A (2012) Una Versione Abbreviata Del Test Di Stroop: Dati Normativi Nella Popolazione Italiana. Nuova Rivista Di Neurologia 12(4):111–115
Basso A, Capitani E, Laiacona M (1987) Raven’s coloured Progressive matrices: normative values on 305 adult normal controls. Funct Neurol 2(2):189–194
Spinnler H, Tognoni G (1987) Italian standardization and classification of neuropsychological tests. The Italian Group on the Neuropsychological Study of Aging. Ital J Neurol Sci ;Suppl 8:1–120
Laiacona M, Inzaghi MG, De Tanti A, Capitani E (2000) Wisconsin card sorting test: a new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol 21(5):279–291. https://doi.org/10.1007/s100720070065
Zarino B, Crespi M, Launi M, Casarotti A (2013) A new standardization of semantic verbal fluency test. Neurol Sci 35(9):1405–1411. https://doi.org/10.1007/s10072-014-1729-1
Catricalà E, Della Rosa PA, Ginex V, Mussetti Z, Plebani V, Cappa SF (2013) An Italian Battery for the assessment of semantic memory disorders. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol 34(6):985–993. https://doi.org/10.1007/s10072-012-1181-z
Papagno C, Casarotti A, Zarino B, Crepaldi D (2020) A new test of action verb naming: normative data from 290 Italian adults. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol 41(10):2811–2817. https://doi.org/10.1007/s10072-020-04353-1
De Renzi E, Faglioni P (1978) Normative data and screening power of a shortened version of the Token Test. Cortex J Devoted Study Nerv Syst Behav 14(1):41–49. https://doi.org/10.1016/s0010-9452(78)80006-9
Novelli G, Papagno C, Capitani E, Laiacona M et al (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. [Three clinical tests to research and rate the lexical performance of normal subjects]. Arch Psicol Neurol Psichiatr 47(4):477–506
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309. https://doi.org/10.1007/BF01997792
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW Studies of Illness in the aged: the Index of ADL: a standardized measure of biological and psychosocial function (1963). J Am Med Associ., 185(12):914–919. https://doi.org/10.1001/jama.1963.03060120024016
Lawton MP, Brody EM Assessment of older people: self-maintaining and instrumental activities of daily living (1969). Gerontologist, 9(3):179–186
Attarian F, Taghizadeh-Hesary F, Fanipakdel A, Javadinia SA, Porouhan P, PeyroShabany B, Fazilat-Panah D (2021) A systematic review and meta-analysis on the number of adjuvant temozolomide cycles in newly diagnosed glioblastoma. Front Oncol 11:779491. https://doi.org/10.3389/fonc.2021.779491
Casarotti A, Papagno C et al (2014) Modified Taylor Complex figure: normative data from 290 adults. J Neuropsychol 8(2):186–198. https://doi.org/10.1111/jnp.12019
Rosso L, Brock CS, Gallo JM, Saleem A, Price PM, Turkheimer FE, Aboagye EO (2009) A new model for prediction of Drug Distribution in Tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res 69(1):120–127. https://doi.org/10.1158/0008-5472.CAN-08-2356
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by G.A.F. and P. B. S. The main manuscript was written by G.A.F. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational restrospective study; our Research Ethics Committee has confirmed that no ethical approval is required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abete-Fornara, G., Bintintan Socaciu, P., Fanizzi, C. et al. Neuropsychological functioning during chemotherapy with temozolomide in high-grade glioma patients: a retrospective single centre study. J Neurooncol 165, 561–568 (2023). https://doi.org/10.1007/s11060-023-04533-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04533-x