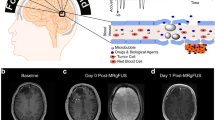
Abstract
Background
Despite advances in immunotherapy and targeted treatments for malignancies of the central nervous system (CNS), the treatment of brain metastases (BMs) remains a formidable challenge, due largely to difficulties in crossing the blood-brain barrier (BBB), drug resistance, and molecular discrepancies. Focused ultrasound (FUS) is a non-invasive tool for BBB breaching, tumor ablation, enhancing drug delivery, promoting the release of tumor biomarkers for liquid biopsy, or the tumor microenvironment disruption. This paper presents a comprehensive review of the current literature related to FUS and its application in the treatment of brain metastasis.
Methods
This review of the current literature via PubMed, Google Scholar, and Clincaltrials.gov focused on clinical trials in which FUS is used in the intracranial treatment of metastatic tumor, glioma, or GBM.
Results
FUS is safe and effective for treatment of primary or metastatic brain tumors. FUS-augmented drug delivery can open BBB to facilitate the transport of chemotherapeutic agents, immunotherapies, and targeted treatments. The integration of FUS with liquid biopsy has considerable potential for early tumor detection, precise gene profiling, and personalized therapy. Sonodynamic therapy can induce tumor cell apoptosis and could potentially be used to enhance the outcomes of other tumor treatments, such as surgery and chemotherapy.
Conclusion
Further work is required to establish FUS as a standard therapy for BMs. FUS has the potential to transform brain tumor treatment, particularly when combined with immunotherapy and targeted therapy as a non-invasive alternative to surgery and radiation therapy.
Similar content being viewed by others
References
Di Giacomo AM et al (2023) Immunotherapy for brain metastases and primary brain tumors. Eur J Cancer 179:113–120. https://doi.org/10.1016/j.ejca.2022.11.012
Mo F, Pellerino A, Soffietti R, Ruda R (2021) Blood-brain barrier in Brain tumors: Biology and Clinical Relevance. Int J Mol Sci 22(23):12654. https://doi.org/10.3390/ijms222312654
Cacho-Diaz B, Garcia-Botello DR, Wegman-Ostrosky T, Reyes-Soto G, Ortiz-Sanchez E, Herrera-Montalvo LA (2020) Tumor microenvironment differences between primary Tumor and brain metastases. J Transl Med 18(1):1. https://doi.org/10.1186/s12967-019-02189-8
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 220(3):640–646. https://doi.org/10.1148/radiol.2202001804
Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N (2005) Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 24(1):12–20. https://doi.org/10.1016/j.neuroimage.2004.06.046
Cohen-Inbar O, Xu Z, Sheehan JP (2016) Focused ultrasound-aided immunomodulation in Glioblastoma Multiforme: a therapeutic concept. J Ther Ultrasound 4:2. https://doi.org/10.1186/s40349-016-0046-y
Yumita N, Nishigaki R, Umemura K, Umemura S (1989) Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res., 80(3):219 – 22. https://doi.org/10.1111/j.1349-7006.1989.tb02295.x
Zhu L et al (2018) Focused ultrasound-enabled Brain Tumor Liquid Biopsy. Sci Rep 8(1):6553. https://doi.org/10.1038/s41598-018-24516-7
Patrick JT, Nolting MN, Goss SA, Dines KA, Clendenon JL, Rea MA, Heimburger RF (1990) Ultrasound and the blood-brain barrier. Adv Exp Med Biol 267:369–381. https://doi.org/10.1007/978-1-4684-5766-7_36
Elhelf IAS, Albahar H, Shah U, Oto A, Cressman E, Almekkawy M (2018) High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging 99(6):349–359. https://doi.org/10.1016/j.diii.2018.03.001
Haar GT, Coussios C (2007) High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 23(2):89–104. https://doi.org/10.1080/02656730601186138
Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA (2010) Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol 36(2):250–267. https://doi.org/10.1016/j.ultrasmedbio.2009.09.010
Vykhodtseva NI, Hynynen K, Damianou C (1995) Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol 21(7):969–979. https://doi.org/10.1016/0301-5629(95)00038-s
Meng Y et al (2019) Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J Control Release 309:25–36. https://doi.org/10.1016/j.jconrel.2019.07.023
Nance E et al (2014) Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J Control Release 189:123–132. https://doi.org/10.1016/j.jconrel.2014.06.031
Huang Y, Alkins R, Schwartz ML, Hynynen K (2017) Opening the blood-brain barrier with MR Imaging-guided focused Ultrasound: preclinical testing on a trans-human Skull Porcine Model. Radiology 282(1):123–130. https://doi.org/10.1148/radiol.2016152154
Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE (2014) Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol 130–137. https://doi.org/10.1016/j.ultrasmedbio.2013.09.015
SK, Wu et al (2017) Characterization of different microbubbles in assisting focused Ultrasound-Induced blood-brain barrier opening. Sci Rep 7:46689. https://doi.org/10.1038/srep46689
McMahon D, O’Reilly MA, Hynynen K (2021) Therapeutic Agent Delivery across the blood-brain barrier using focused Ultrasound. Annu Rev Biomed Eng 23:89–113. https://doi.org/10.1146/annurev-bioeng-062117-121238
Liu HL et al (2008) Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med Biol 34(4):598–606. https://doi.org/10.1016/j.ultrasmedbio.2008.01.011
Anastasiadis P et al (2021) Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci U S A 118(37):e2103280118. https://doi.org/10.1073/pnas.2103280118
KT, Chen et al (2020) Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: clinical trial protocol. Ann Transl Med 8(11):673. https://doi.org/10.21037/atm-20-344
KT, Chen et al (2021) Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv 7(6):eabd0772. https://doi.org/10.1126/sciadv.abd0772
Carpentier A et al (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8(343):343re2. https://doi.org/10.1126/scitranslmed.aaf6086
Yonemori K et al (2010) Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type Breast cancer but not HER2/neu-positive Breast cancer. Cancer 116(2):302–308. https://doi.org/10.1002/cncr.24735
Lipsman N et al (2018) Blood-brain barrier opening in Alzheimer’s Disease using MR-guided focused ultrasound. Nat Commun 9(1):2336. https://doi.org/10.1038/s41467-018-04529-6
Meng Y et al (2021) MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci Transl Med 13(615):eabj4011. https://doi.org/10.1126/scitranslmed.abj4011
Park SH et al (2020) One-year outcome of multiple blood-brain barrier disruptions with Temozolomide for the treatment of Glioblastoma. Front Oncol 10:1663. https://doi.org/10.3389/fonc.2020.01663
Ahluwalia MS, Becker K, Levy BP (2018) Epidermal growth factor receptor tyrosine kinase inhibitors for Central Nervous System metastases from Non-small Cell Lung Cancer. Oncologist 23(10):1199–1209. https://doi.org/10.1634/theoncologist.2017-0572
Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K (2007) Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer 121(4):901–907. https://doi.org/10.1002/ijc.22732
Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K (2012) Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 38(10):1716–1725. https://doi.org/10.1016/j.ultrasmedbio.2012.04.015
Aryal M, Vykhodtseva N, Zhang YZ, McDannold N (2015) Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: a safety study. J Control Release 204:60–69. https://doi.org/10.1016/j.jconrel.2015.02.033
Kinoshita M, McDannold N, Jolesz FA, Hynynen K (2006) Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 103(31):11719–11723. https://doi.org/10.1073/pnas.0604318103
Park EJ, Zhang YZ, Vykhodtseva N, McDannold N (2012) Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a Breast cancer brain Metastasis model. J Control Release 163(3):277–284. https://doi.org/10.1016/j.jconrel.2012.09.007
HL, Liu et al (2016) Focused Ultrasound enhances Central Nervous System Delivery of Bevacizumab for malignant glioma treatment. Radiology 281(1):99–108. https://doi.org/10.1148/radiol.2016152444
Rincon-Torroella J, Khela H, Bettegowda A, Bettegowda C (2022) Biomarkers and focused ultrasound: the future of liquid biopsy for Brain Tumor patients. J Neurooncol 156(1):33–48. https://doi.org/10.1007/s11060-021-03837-0
Liu HL, Huang CY, Chen JY, Wang HY, Chen PY, Wei KC (2014) Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS ONE 9(12):e114311. https://doi.org/10.1371/journal.pone.0114311
KC Wei et al et al (2013) Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS ONE 8(3):e58995. https://doi.org/10.1371/journal.pone.0058995
Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, Wang Z (2009) Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med 28(7):871–880. https://doi.org/10.7863/jum.2009.28.7.871
Dréan A et al (2019) Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J Neurooncol 144(1):33–41. https://doi.org/10.1007/s11060-019-03204-0
Zhang S et al (2022) Ultrasound-assisted brain delivery of nanomedicines for Brain Tumor therapy: advance and prospect. J Nanobiotechnol 20(1):287. https://doi.org/10.1186/s12951-022-01464-z
Idbaih A et al (2019) Safety and feasibility of repeated and transient blood-brain barrier disruption by Pulsed Ultrasound in patients with recurrent glioblastoma. Clin Cancer Res 25(13):3793–3801. https://doi.org/10.1158/1078-0432.CCR-18-3643
Sonabend AM et al (2023) Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: a phase 1 trial. Lancet Oncol 24(5):509–522. https://doi.org/10.1016/S1470-2045(23)00112-2
Parekh K et al (2023) Past, present and future of focused Ultrasound as an adjunct or complement to DIPG/DMG therapy: a consensus of the 2021 FUSF DIPG meeting. Neoplasia 37:100876. https://doi.org/10.1016/j.neo.2023.100876
AL, D’Souza et al (2009) A strategy for blood biomarker amplification and localization using ultrasound. Proc Natl Acad Sci U S A 106(40):17152–17157. https://doi.org/10.1073/pnas.0903437106
Zhu L, Nazeri A, Pacia CP, Yue Y, Chen H (2020) Focused ultrasound for safe and effective release of Brain Tumor biomarkers into the peripheral circulation. PLoS ONE 15(6):e0234182. https://doi.org/10.1371/journal.pone.0234182
CP, Pacia et al (2022) Sonobiopsy for minimally invasive, spatiotemporally-controlled, and sensitive detection of glioblastoma-derived circulating Tumor DNA. Theranostics 12(1):362–378. https://doi.org/10.7150/thno.65597
CP, Pacia et al (2020) Feasibility and safety of focused ultrasound-enabled liquid biopsy in the brain of a porcine model. Sci Rep 10(1):7449. https://doi.org/10.1038/s41598-020-64440-3
Meng Y et al (2021) MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol 23(10):1789–1797. https://doi.org/10.1093/neuonc/noab057
Katzendobler S et al (2022) The value of stereotactic biopsy of primary and recurrent brain metastases in the era of precision medicine. Front Oncol 12:1014711. https://doi.org/10.3389/fonc.2022.1014711
Bonosi L et al (2023) Sonodynamic therapy and magnetic resonance-guided focused ultrasound: new therapeutic strategy in glioblastoma. J Neurooncol 163(1):219–238. https://doi.org/10.1007/s11060-023-04333-3
Sheehan D, Sheehan K, Sheehan J (2021) Sonodynamic therapy for metastatic Melanoma to the brain. J Neurooncol 153(2):373–374. https://doi.org/10.1007/s11060-021-03768-w
HR, Syed et al (2023) First-in-human sonodynamic therapy with ALA for pediatric diffuse intrinsic pontine glioma: a phase 1/2 study using low-intensity focused ultrasound: technical communication. J Neurooncol 162(2):449–451. https://doi.org/10.1007/s11060-023-04269-8
TA, Arsiwala et al (2023) Blood-tumor barrier opening by MRI-guided transcranial focused ultrasound in a preclinical Breast cancer brain Metastasis model improves efficacy of combinatorial chemotherapy. Front Oncol 13:1104594. https://doi.org/10.3389/fonc.2023.1104594
Joiner JB, Pylayeva-Gupta Y, Dayton PA (2020) Focused Ultrasound for Immunomodulation of the Tumor Microenvironment. J Immunol 205(9):2327–2341. https://doi.org/10.4049/jimmunol.1901430
Zhang Y, Deng J, Feng J, Wu F (2010) Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol 16(28):3584–3591. https://doi.org/10.3748/wjg.v16.i28.3584
Suzuki R et al (2016) Tumor growth suppression by the combination of nanobubbles and ultrasound. Cancer Sci 107(3):217–223. https://doi.org/10.1111/cas.12867
Bandyopadhyay S et al (2016) Low-intensity focused Ultrasound induces reversal of Tumor-Induced T Cell Tolerance and prevents Immune Escape. J Immunol 196(4):1964–1976. https://doi.org/10.4049/jimmunol.1500541
JR, Sukovich et al (2018) In vivo histotripsy brain treatment. J Neurosurg 1–8. https://doi.org/10.3171/2018.4.JNS172652
KM, Imran et al (2023) Magic bubbles: utilizing histotripsy to modulate the Tumor microenvironment and improve systemic anti-tumor immune responses. Int J Hyperthermia 40(1):2244206. https://doi.org/10.1080/02656736.2023.2244206
Xing Y, Lu X, Pua EC, Zhong P (2008) The effect of high intensity focused ultrasound treatment on metastases in a murine Melanoma model. Biochem Biophys Res Commun 375(4):645–650. https://doi.org/10.1016/j.bbrc.2008.08.072
Funding
This study was supported by Taipei Veterans General Hospital (VGHUST112-G1-3-1 and V112C-105). The sponsor had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
The study conception and design were done by YHS and CCL. Data acquisition and analysis were done by YHC, CCL and DM. The manuscript writing was done by YHC and YHS. Critical revision and final approval of the manuscript were done by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Compliance with ethical standards
Not applicable.
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YH., Moore, D., Lee, CC. et al. Focused ultrasound for brain metastases: an update on global clinical trials. J Neurooncol 165, 53–62 (2023). https://doi.org/10.1007/s11060-023-04492-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04492-3