Abstract
Purpose
Distinguishing radiation necrosis from tumor progression among patients with brain metastases previously treated with stereotactic radiosurgery represents a common diagnostic challenge. We performed a prospective pilot study to determine whether PET/CT with 18F-fluciclovine, a widely available amino acid PET radiotracer, repurposed intracranially, can accurately diagnose equivocal lesions.
Methods
Adults with brain metastases previously treated with radiosurgery presenting with a follow-up tumor-protocol MRI brain equivocal for radiation necrosis versus tumor progression underwent an 18F-fluciclovine PET/CT of the brain within 30 days. The reference standard for final diagnosis consisted of clinical follow-up until multidisciplinary consensus or tissue confirmation.
Results
Of 16 patients imaged from 7/2019 to 11/2020, 15 subjects were evaluable with 20 lesions (radiation necrosis, n = 16; tumor progression, n = 4). Higher SUVmax statistically significantly predicted tumor progression (AUC = 0.875; p = 0.011). Lesion SUVmean (AUC = 0.875; p = 0.018), SUVpeak (AUC = 0.813; p = 0.007), and SUVpeak-to-normal-brain (AUC = 0.859; p = 0.002) also predicted tumor progression, whereas SUVmax-to-normal-brain (p = 0.1) and SUVmean-to-normal-brain (p = 0.5) did not. Qualitative visual scores were significant predictors for readers 1 (AUC = 0.750; p < 0.001) and 3 (AUC = 0.781; p = 0.045), but not for reader 2 (p = 0.3). Visual interpretations were significant predictors for reader 1 (AUC = 0.898; p = 0.012) but not for reader 2 (p = 0.3) or 3 (p = 0.2).
Conclusions
In this prospective pilot study of patients with brain metastases previously treated with radiosurgery presenting with a contemporary MRI brain with a lesion equivocal for radiation necrosis versus tumor progression, 18F-fluciclovine PET/CT repurposed intracranially demonstrated encouraging diagnostic accuracy, supporting the pursuit of larger clinical trials which will be necessary to establish diagnostic criteria and performance.


Similar content being viewed by others
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
Lehrer EJ et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103(3):618–630
Brown PD et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316(4):401–409
Patel TR et al (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 32(10):1885–1892
Chao ST et al (2013) Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys 87(3):449–457
Walker AJ et al (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 10(7):1277–1297
Borghei-Razavi H et al (2020) Pathologic correlation of cellular imaging using apparent diffusion coefficient quantification in patients with brain metastases after gamma knife radiosurgery. World Neurosurg 134:e903–e912
Galldiks N et al (2019) PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol 21(5):585–595
Tsuyuguchi N et al (2003) Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg 98(5):1056–1064
Terakawa Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49(5):694–699
Minamimoto R et al (2015) Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS One 10(7):e0132515
Tomura N et al (2017) Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among (11)C-methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol 38(8):1520–1527
Yomo S, Oguchi K (2017) Prospective study of (11)C-methionine PET for distinguishing between recurrent brain metastases and radiation necrosis: limitations of diagnostic accuracy and long-term results of salvage treatment. BMC Cancer 17(1):713
Lizarraga KJ et al (2014) (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 55(1):30–36
Cicone F et al (2015) Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging 42(1):103–111
Galldiks N et al (2012) Role of O-(2-(18)F-fluoroethyl)-l-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53(9):1367–1374
Romagna A et al (2016) Suspected recurrence of brain metastases after focused high dose radiotherapy: can [(18)F]FET-PET overcome diagnostic uncertainties? Radiat Oncol 11(1):139
Ceccon G et al (2017) Dynamic O-(2–18F-fluoroethyl)-l-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol 19(2):281–288
Parent EE, Schuster DM (2018) Update on (18)F-fluciclovine PET for prostate cancer imaging. J Nucl Med 59(5):733–739
Michaud L et al (2020) (18)F-Fluciclovine ((18)F-FACBC) PET imaging of recurrent brain tumors. Eur J Nucl Med Mol Imaging 47(6):1353–1367
Bogsrud TV et al (2019) 18F-fluciclovine PET/CT in suspected residual or recurrent high-grade glioma. Clin Nucl Med 44(8):605–611
Karlberg A et al (2019) 18F-FACBC PET/MRI in diagnostic assessment and neurosurgery of gliomas. Clin Nucl Med 44(7):550–559
Parent EE et al (2018) [(18)F]Fluciclovine PET discrimination between high- and low-grade gliomas. EJNMMI Res 8(1):67
Tsuyuguchi N et al (2017) Diagnosis of brain tumors using amino acid transport PET imaging with (18)F-fluciclovine: a comparative study with l-methyl-(11)C-methionine PET imaging. Asia Ocean J Nucl Med Biol 5(2):85–94
Wakabayashi T et al (2017) Diagnostic performance and safety of positron emission tomography using (18)F-fluciclovine in patients with clinically suspected high- or low-grade gliomas: a multicenter phase IIb trial. Asia Ocean J Nucl Med Biol 5(1):10–21
Kondo A et al (2016) Phase IIa clinical study of [(18)F]fluciclovine: efficacy and safety of a new PET tracer for brain tumors. Ann Nucl Med 30(9):608–618
Henderson F Jr et al (2020) (18)F-Fluciclovine PET to distinguish treatment-related effects from disease progression in recurrent glioblastoma: PET fusion with MRI guides neurosurgical sampling. Neurooncol Pract 7(2):152–157
Karlberg A et al (2017) Multimodal (18)F-fluciclovine PET/MRI and ultrasound-guided neurosurgery of an anaplastic oligodendroglioma. World Neurosurg 108:989 e1-989 e8
Ono M et al (2013) Comparative evaluation of transport mechanisms of trans-1-amino-3-[(1)(8)F]fluorocyclobutanecarboxylic acid and l-[methyl-(1)(1)C]methionine in human glioma cell lines. Brain Res 1535:24–37
Johannessen K et al (2021) (18)F-FACBC PET/MRI in the evaluation of human brain metastases: a case report. Eur J Hybrid Imaging 5(1):7
Parent EE et al (2020) [(18)F]-Fluciclovine PET discrimination of recurrent intracranial metastatic disease from radiation necrosis. EJNMMI Res 10(1):148
Wahl RL et al (2009) From RECIST to PERCIST: evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S-S150
Lin NU et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270–e278
Bai B, Bading J, Conti PS (2013) Tumor quantification in clinical positron emission tomography. Theranostics 3(10):787–801
Aklan B et al (2016) Impact of point-spread function modeling on pet image quality in integrated PET/MR hybrid imaging. J Nucl Med 57(1):78–84
Acknowledgements
The authors would like to thank Robin Davis for data support for this study and philanthropic support by the Kerscher family.
Funding
Cleveland Clinic Department of Radiation Oncology, Cleveland Clinic Lerner Research Institute (Research Programs Committee Grant #326), Cleveland Clinic Imaging Institute, and Blue Earth Diagnostics.
Author information
Authors and Affiliations
Contributions
MCT, FDP, SEJ, and STC wrote the main manuscript text. NAO: responsible for statistical analysis. All authors reviewed the manusript.
Corresponding author
Ethics declarations
Conflict of interest
MCT: Honoraria from ViewRay. Institutional research funding from Blue Earth Diagnostics Ltd. Personal fees from Elsevier. FPD: None. SEJ: None. JHS: Scientific advisory board for and consulting fees from Philips, Novocure. Leadership role with American Board of Radiology, International Radiosurgery Research Foundation, International Stereotactic Radiosurgery Society. NAO: Grants or contracts from Quantitative Imaging Biomarker Alliance. Honoraria from NCI Clinical Imaging Steering committee and ECOG-ACRIN DBMB. TDS: None. ESM: None. JSY: None. GHB: Monteris Medical, Inc. Plymouth, Minnesota. LA: None. AMM: None. SSH: None. GW: None. SJ: None. DMP: None. GHJS: None. MSA: Receipt of grants/research supports: Astrazeneca, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Mimivax, Merck. Receipt of honoraria or consultation fees: Bayer, Novocure, Kiyatec, Insightec, GSK, Xoft, Nuvation, Cellularity, SDP Oncology, Apollomics, Prelude, Janssen, Tocagen, Voyager Therapeutics, Viewray, AnHeart Therapeutics, Caris Lifesciences, Theraguix, Pyramid biosciences. Scientific advisory board: Modifi bio, Pyramid Biosciences, Cairn Therapeutics, Bugworks. Stock shareholder: Doctible, Mimivax, Cytodyn, MedInnovate Advisors LLC. STC: Honoraria from Varian Medical Systems and Blue Earth Diagnostics. Travel support from Blue Earth Diagnostics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11060_2023_4377_MOESM1_ESM.pdf
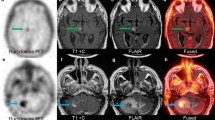
Supplemental Fig. 1. Representative Cases. The upper panels depict imaging for Patient A showing new enhancement after SRS and associated 18F-fluciclovine PET/CT demonstrating low uptake. Biopsy returned radiation necrosis. The lower panels depict imaging for Patient B showing new enhancement after SRS and associated 18F-fluciclovine PET/CT demonstrating high uptake. Biopsy returned viable tumor as indicated by the arrow. (PDF 1221 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tom, M.C., DiFilippo, F.P., Jones, S.E. et al. 18F-fluciclovine PET/CT to distinguish radiation necrosis from tumor progression for brain metastases treated with radiosurgery: results of a prospective pilot study. J Neurooncol 163, 647–655 (2023). https://doi.org/10.1007/s11060-023-04377-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04377-5