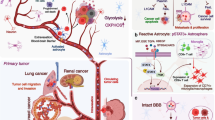
Abstract
Acquired mutations or altered gene expression patterns in brain metastases (BM) and/or leptomeningeal metastases (LM) of breast cancer may play a role in therapy-resistance and offer new molecular targets and treatment options. Despite expanding knowledge of genetic alterations in breast cancer and their metastases, clinical applications for patients with central nervous system (CNS) metastases are currently limited. An emerging tool are DNA-techniques that may detect genetic alterations of the CNS metastases in the cerebrospinal fluid (CSF). In this review we discuss genetic studies in breast cancer and CNS metastases and the role of liquid biopsies in CSF.


Similar content being viewed by others
References
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13:1648–1655
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617
Wang N, Bertalan MS, Brastianos PK (2018) Leptomeningeal metastasis from systemic cancer: review and update on management. Cancer 124:21–35. https://doi.org/10.1002/cncr.30911
Schrijver WAME, Suijkerbuijk KPM, Van Gils CH et al (2018) Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst 110:568–580
Hulsbergen AFC, Claes A, Kavouridis VK et al (2020) Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa013
Cardoso F, Senkus E, Costa A et al (2018) 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol 29:1634–1657. https://doi.org/10.1093/annonc/mdy192
Lin NU, Borges V, Anders C et al (2020) Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 38:2610–2619. https://doi.org/10.1200/JCO.20.00775
Pérez-García JM, Batista MV, Cortez P et al (2022) Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. https://doi.org/10.1093/NEUONC/NOAC144
Brastianos PK, Carter SL, Santagata S et al (2015) Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. https://doi.org/10.1158/2159-8290.CD-15-0369
Turashvili G, Brogi E (2017) Tumor heterogeneity in breast cancer. Front Med 4:227. https://doi.org/10.3389/fmed.2017.00227
Tarin D (2008) Comparisons of metastases in different organs: biological and clinical implications. Clin Cancer Res 14:1923–1925
Morgan AJ, Giannoudis A, Palmieri C (2021) The genomic landscape of breast cancer brain metastases: a systematic review. Lancet Oncol. https://doi.org/10.1016/S1470-2045(20)30556-8
Bertucci F, Ng CKY, Patsouris A et al (2019) Genomic characterization of metastatic breast cancers. Nature 569:560–564. https://doi.org/10.1038/s41586-019-1056-z
Angus L, Smid M, Wilting SM et al (2019) The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat Genet 51:1450–1458. https://doi.org/10.1038/s41588-019-0507-7
Huang RSP, Haberberger J, Mcgregor K et al (2021) Clinicopathologic and genomic landscape of breast carcinoma brain metastases. Oncologist. https://doi.org/10.1002/onco.13855
Giannoudis A, Sartori A, Eastoe L et al (2021) Genomic profiling using the UltraSEEK panel identifies discordancy between paired primary and breast cancer brain metastases and an association with brain metastasis-free survival. Breast Cancer Res Treat 190:241–253. https://doi.org/10.1007/S10549-021-06364-8
Da Silva L, Simpson PT, Smart CE et al (2010) HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res 12:R46. https://doi.org/10.1186/bcr2603
Wikman H, Lamszus K, Detels N et al (2012) Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res 14:R49. https://doi.org/10.1186/BCR3150
Lee JY, Park K, Lim SH et al (2015) Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget 6:43731–43742. https://doi.org/10.18632/oncotarget.6192
Bollig-Fischer A, Michelhaugh SK, Wijesinghe P et al (2015) Cytogenomic profiling of breast cancer brain metastases reveals potential for repurposing targeted therapeutics. Oncotarget 6:14614–14624. https://doi.org/10.18632/oncotarget.3786
Schrijver WA, Selenica P, Lee JY et al (2018) Mutation profiling of key cancer genes in primary breast cancers and their distant metastases. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-17-2310
De Mattos-Arruda L, Ng CKY, Piscuoglio S et al (2018) Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases. Oncotarget 9:20617–20630. https://doi.org/10.18632/oncotarget.25041
Tyran M, Carbuccia N, Garnier S et al (2019) A comparison of DNA mutation and copy number profiles of primary breast cancers and paired brain metastases for identifying clinically relevant genetic alterations in brain metastases. Cancers (Basel). https://doi.org/10.3390/CANCERS11050665
Thulin A, Andersson C, Werner Rönnerman E et al (2021) Discordance of PIK3CA and TP53 mutations between breast cancer brain metastases and matched primary tumors. Sci Rep 11:23548. https://doi.org/10.1038/s41598-021-02903-x
Gao R, Davis A, McDonald TO et al (2016) Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet 48:1119–1130. https://doi.org/10.1038/ng.3641
Magbanua MJM, Melisko M, Roy R et al (2013) Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res 73:7134–7143. https://doi.org/10.1158/0008-5472.CAN-13-2051
De M-A, Mayor R, Ng CKY et al (2015) Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. https://doi.org/10.1038/ncomms9839
White MD, Klein RH, Shaw B et al (2021) Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2021.20040
Fitzpatrick A, Iravani M, Mills A et al (2022) Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin Cancer Res 28:1180–1191. https://doi.org/10.1158/1078-0432.CCR-21-3017
Boire A, Brandsma D, Brastianos PK et al (2019) Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 21:571–583. https://doi.org/10.1093/neuonc/noz012
Cheok SK, Narayan A, Arnal-Estape A et al (2021) Tumor DNA mutations from intraparenchymal brain metastases are detectable in CSF. JCO Precis Oncol. https://doi.org/10.1200/PO.20.00292
Lee JH, Menzies AM, Carlino MS et al (2020) Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-3926
Pentsova EI, Shah RH, Tang J et al (2016) Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol 34:2404–2415. https://doi.org/10.1200/JCO.2016.66.6487
Ma C, Yang X, Xing W et al (2020) Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thoracic Cancer. https://doi.org/10.1111/1759-7714.13300
von Baumgarten L, Kumbrink J, Jung A et al (2020) Therapeutic management of neuro-oncologic patients—potential relevance of CSF liquid biopsy. Theranostics 10:856–866. https://doi.org/10.7150/thno.36884
Siravegna G, Geuna E, Mussolin B et al (2017) Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open 2:e00253. https://doi.org/10.1136/esmoopen-2017-000253
Li X, Zhang Y, Ding J et al (2018) Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget. https://doi.org/10.18632/oncotarget.23597
Carausu M, Melaabi S, Pierga J-Y et al (2020) ESR1 mutation detection and dynamics in meningeal carcinomatosis in breast cancer. J Breast Cancer 23:218. https://doi.org/10.4048/jbc.2020.23.e4
Angus L, Deger T, Jager A et al (2021) Detection of aneuploidy in cerebrospinal fluid from patients with breast cancer can improve diagnosis of leptomeningeal metastases. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-3954
Shah M, Takayasu T, Zorofchian Moghadamtousi S et al (2021) Evaluation of the oncomine pan-cancer cell-free assay for analyzing circulating tumor DNA in the cerebrospinal fluid in patients with central nervous system malignancies. J Mol Diagnostics 23:171–180. https://doi.org/10.1016/j.jmoldx.2020.10.013
Chandarlapaty S, Chen D, He W et al (2016) Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer. JAMA Oncol 2:1310. https://doi.org/10.1001/jamaoncol.2016.1279
Razavi P, Chang MT, Xu G et al (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34:427-438.e6. https://doi.org/10.1016/J.CCELL.2018.08.008
Fitzgerald DM, Muzikansky A, Pinto C et al (2019) Association between PIK3CA mutation status and development of brain metastases in HR+/HER2− metastatic breast cancer. Ann Oncol. https://doi.org/10.1093/annonc/mdz242.013
Berns K, Horlings HM, Hennessy BT et al (2007) A functional genetic approach identifies the PI3K Pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395–402. https://doi.org/10.1016/j.ccr.2007.08.030
Pascual J, Turner NC (2019) Targeting the PI3-kinase pathway in triple negative breast cancer. Ann Oncol. https://doi.org/10.1093/annonc/mdz133
Batalini F, Moulder SL, Winer EP et al (2020) Response of brain metastases from PIK3CA -Mutant breast cancer to alpelisib. JCO Precis Oncol. https://doi.org/10.1200/po.19.00403
Formisano L, Lu Y, Servetto A et al (2019) Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. https://doi.org/10.1038/S41467-019-09068-2
Meric-Bernstam F, Bahleda R, Hierro C et al (2022) Futibatinib, an irreversible FGFR1-4 Inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase i dose-expansion study. Cancer Discov 12:402–415. https://doi.org/10.1158/2159-8290.CD-21-0697
Kodack DP, Askoxylakis V, Ferraro GB et al (2017) The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. https://doi.org/10.1126/SCITRANSLMED.AAL4682
Saunus J, McCart Reed A, Lim Z, Lakhani S (2017) Breast cancer brain metastases: clonal evolution in clinical context. Int J Mol Sci 18:152. https://doi.org/10.3390/ijms18010152
Seoane J, De Mattos-Arruda L, Le Rhun E et al (2019) Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann Oncol Off J Eur Soc Med Oncol 30:211–218. https://doi.org/10.1093/annonc/mdy544
Pan W, Gu W, Nagpal S et al (2015) Brain tumor mutations detected in cerebral spinal fluid. Clin Chem 61:514–522. https://doi.org/10.1373/clinchem.2014.235457
Funding
The authors have not disclosed any funding.
Ethics declarations
Conflict of interest
PHW and MCB report no conflicts of interest KM received consultant fees from Pfizer, BMS, Roche, MSD, Abbvie, AstraZeneca, Diaceutics, Lilly, Bayer, Boehringer Ingelheim, Merck and a research grant from Astra Zeneca. Non-financial support from Roche, Takeda, Pfizer, PGDx, Delfi and speakers fees from MSD, Roche, Astra Zeneca, Benecke GS received research support paid to the intstitution from Agendia, AstraZeneca, Merck, Novartis, Roche and Seagen DvdB received honoraria for lectures, presentations, manuscript writing or educational events from Roche Diagnostics DB has an advisory role for Lilly Pharmaceuticals and received institutional research grants from Bristol-Myers Squibb
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wessels, P.H., Boelens, M.C., Monkhorst, K. et al. A review on genetic alterations in CNS metastases related to breast cancer treatment. Is there a role for liquid biopsies in CSF?. J Neurooncol 162, 1–13 (2023). https://doi.org/10.1007/s11060-023-04261-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04261-2