Abstract
Purpose
During intracranial meningioma surgery, surgeons experience considerable blood loss. Tranexamic acid (TXA) is used to minimize blood loss in several neurosurgical settings. However, evidence and trials are lacking. Our objective is to establish the most recent evidence on TXA safety and efficacy in intracranial meningioma surgery.
Methodology
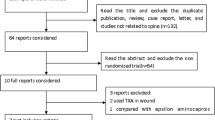
Based upon Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), the authors collected fully published English literature on the administration of tranexamic acid for patients undergoing intracranial meningioma surgery using the keywords [“tranexamic acid” and “meningioma”] and its synonyms from Cochrane Central Database, the WHO International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, and PubMed. The primary outcome of the current study was total blood loss. The secondary outcomes include individuals requiring blood transfusion, anesthesia duration, surgical duration, and complication rate. Each included studies' quality was assessed using the JADAD scale.
Results
For qualitative and quantitative data synthesis, we included five RCTs (n = 321) with the mean age was 47.5 ± 11.9 years for the intervention group and 47.2 ± 11.9 years for the control group. Our meta-analysis showed that the administration of TXA is associated with decreased total blood loss of standardized mean difference (SMD) of −1.40 (95% CI [−2.49, −0.31]), anesthetic time SMD −0.36 (95% CI [−0.63, −0.09]), and blood transfusion requirements RR 0.58 (95% CI [0.34, 0.99]).
Conclusions
The current study showed that TXA was associated with reduced intraoperative blood loss and intra- and postoperative blood transfusion. However, the studies are small. More RCT studies with a greater sample size are favorable.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TXA:
-
Tranexamic acid
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ICTRP:
-
International Clinical Trials Registry Platform
- RR:
-
Risk ratio
- MD:
-
Mean difference
- CI:
-
Confidence interval
- RCT:
-
Randomized controlled trial
- N:
-
Number
- mL:
-
Millilitre
- min:
-
Minute
- mg:
-
Milligram
- kg:
-
Kilogram
- h:
-
Hour
- NS:
-
Normal saline
- vs:
-
Versus
References
Ostrom QT, Cioffi G, Gittleman H et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21:1–100. https://doi.org/10.1093/neuonc/noz150
Fathi A-R, Roelcke U (2013) Meningioma. Curr Neurol Neurosci Rep 13:337. https://doi.org/10.1007/s11910-013-0337-4
Marosi C, Hassler M, Roessler K et al (2008) Meningioma. Crit Rev Oncol Hematol 67:153–171. https://doi.org/10.1016/j.critrevonc.2008.01.010
Cappabianca P, Cavallo LM, Esposito F et al (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg 33:151–199. https://doi.org/10.1007/978-3-211-72283-1_4
Teng Z, Zhu Y, Liu Y et al (2015) Restrictive blood transfusion strategies and associated infection in orthopedic patients: a meta-analysis of 8 randomized controlled trials. Sci Rep 5:13421. https://doi.org/10.1038/srep13421
Maw G, Furyk C (2018) Pediatric massive transfusion: a systematic review. Pediatr Emerg Care 34:594–598. https://doi.org/10.1097/PEC.0000000000001570
Gremmel T, Frelinger AL, Michelson AD (2016) Platelet physiology. Semin Thromb Hemost 42:191–204. https://doi.org/10.1055/s-0035-1564835
Levy JH, Koster A, Quinones QJ et al (2018) Antifibrinolytic therapy and perioperative considerations. Anesthesiology 128:657–670. https://doi.org/10.1097/ALN.0000000000001997
Sigaut S, Tremey B, Ouattara A et al (2014) Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology 120:590–600. https://doi.org/10.1097/ALN.0b013e3182a443e8
Pinosky ML, Kennedy DJ, Fishman RL et al (1997) Tranexamic acid reduces bleeding after cardiopulmonary bypass when compared to epsilon aminocaproic acid and placebo. J Card Surg 12:330–338. https://doi.org/10.1111/j.1540-8191.1997.tb00147.x
Lee SY, Chong S, Balasubramanian D et al (2017) What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res 475:1987–1996. https://doi.org/10.1007/s11999-017-5311-z
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:1–9. https://doi.org/10.1136/BMJ.N71
Rethlefsen ML, Kirtley S, Waffenschmidt S et al (2021) (2021) PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev 101(10):1–19. https://doi.org/10.1186/S13643-020-01542-Z
Wijaya JH, July J, Quintero-Consuegra M (2021) A systematic review and meta-analysis of the effects of tranexamic acid in surgical procedure for intracranial meningioma. PROSPERO 2021 CRD42021272386. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021272386. Accessed 7 Mar 2022
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12. https://doi.org/10.1016/0197-2456(95)00134-4
Hooda B, Chouhan RS, Rath GP et al (2017) Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci 41:132–138. https://doi.org/10.1016/j.jocn.2017.02.053
Sutanto S, Yulianti Bisri D, Bisri T (2019) Pengaruh Asam Traneksamat Intravena terhadap Jumlah Perdarahan Intraoperatif dan Kebutuhan Transfusi pada Operasi Meningioma. J Neuroanestesi Indones 8:8–16. https://doi.org/10.24244/jni.vol8i1.200
Siddiqui A, Raman R, Arshad Z et al (2018) Use of tranexamic acid to reduce intraoperative bleeding in craniotomy for meningioma patients. Asian Arch Anesthesiol Resusc 1:1–13
Rebai L, Mahfoudhi N, Fitouhi N et al (2021) Intraoperative tranexamic acid use in patients undergoing excision of intracranial meningioma: randomized, placebo-controlled trial. Surg Neurol Int 12:289. https://doi.org/10.25259/SNI_177_2021
Ravi GK, Panda N, Ahluwalia J et al (2021) Effect of tranexamic acid on blood loss, coagulation profile, and quality of surgical field in intracranial meningioma resection: a prospective randomized, double-blind, placebo-controlled study. Surg Neurol Int 12:272. https://doi.org/10.25259/SNI_296_2021
Lecker I, Wang D-S, Whissell PD et al (2016) Tranexamic acid-associated seizures: causes and treatment. Ann Neurol 79:18–26. https://doi.org/10.1002/ana.24558
Schwinn DA, Mackensen GB, Brown EN (2012) Understanding the TXA seizure connection. J Clin Invest 122:4339–4341. https://doi.org/10.1172/JCI66724
Hvas A-M, Sørensen HT, Norengaard L et al (2007) Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J Thromb Haemost 5:2408–2414. https://doi.org/10.1111/j.1538-7836.2007.02755.x
Ker K, Prieto-Merino D, Roberts I (2013) Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg 100:1271–1279. https://doi.org/10.1002/bjs.9193
Zufferey PJ, Lanoiselée J, Graouch B et al (2021) Exposure-response relationship of tranexamic acid in cardiac surgery. Anesthesiology 134:165–178. https://doi.org/10.1097/ALN.0000000000003633
Heyns M, Knight P, Steve AK, Yeung JK (2021) A Single Preoperative dose of tranexamic acid reduces perioperative blood loss: a meta-analysis. Ann Surg 273:75–81. https://doi.org/10.1097/SLA.0000000000003793
Vel R, Udupi BP, Satya Prakash MVS et al (2015) Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth 9:42–48. https://doi.org/10.4103/1658-354X.146304
Dadure C, Sauter M, Bringuier S et al (2011) Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology 114:856–861. https://doi.org/10.1097/ALN.0b013e318210f9e3
CRASH-2 trial collaborators, Shakur H, Roberts I et al (2010) Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet (London, England) 376:23–32. https://doi.org/10.1016/S0140-6736(10)60835-5
Dewan Y, Komolafe EO, Mejía-Mantilla JH et al (2012) CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials 13:87. https://doi.org/10.1186/1745-6215-13-87
Baharoglu MI, Germans MR, Rinkel GJ et al (2013) Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001245.pub2
Brown NJ, Wilson B, Ong V et al (2022) Use of tranexamic acid for elective resection of intracranial neoplasms: a systematic review. World Neurosurg. https://doi.org/10.1016/j.wneu.2021.12.117
Li S, Yan X, Li R et al (2022) Safety of intravenous tranexamic acid in patients undergoing supratentorial meningiomas resection: protocol for a randomised, parallel-group, placebo control, non-inferiority trial. BMJ Open 12:e052095. https://doi.org/10.1136/bmjopen-2021-052095
Pin P (2020) Tranexamic acid reduce blood loss in meningioma resection. In: ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04386642?cond=tranexamic+acid+and+meningioma&draw=2&rank=2 . Accessed 7 Mar 2022
Peng Y (2020) The safety of intravenous tranexamic acid in patients undergoing supratentorial meningiomas resection (STAMP). In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04595786?cond=tranexamic+acid+and+meningioma&draw=2&rank=3 Accessed 7 Mar 2022
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JHW, JJ, MQC, and DPC. The first draft of the manuscript was written by JHW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wijaya, J.H., July, J., Quintero-Consuegra, M. et al. A systematic review and meta-analysis of the effects of tranexamic acid in surgical procedure for intracranial meningioma. J Neurooncol 161, 383–393 (2023). https://doi.org/10.1007/s11060-023-04237-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04237-2