Abstract
Object
Hemangioblastoma is a relatively rare neoplasm occurring mostly in the cerebellum that may arise sporadically or in the context of von Hippel–Lindau (VHL) syndrome. Presentation, imaging, natural history, surgical patterns of care, and outcomes are incompletely defined for this uncommon lesion. We reviewed our large institutional series to help clarify these issues.
Methods
Retrospective analysis of consecutive, neurosurgically managed CNS hemangioblastomas at Mayo Clinic, 1988–2018.
Results
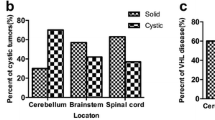
Two hundred and eighty five hemangioblastomas were treated in 184 unique patients (115 sporadic, 69 VHL). Compared to sporadic patients, VHL patients were younger (36.7 vs 51.7 years; p < 0.0001), were treated while asymptomatic more commonly (47.3 vs 4.2%; p < 0.0001), had smaller lesions (6.6 vs 13.9 mL; p < 0.0001), and harbored lesions with associated cysts less frequently (51.0 vs 75.0%; p = 0.0002). Macrocystic tumor architecture was associated with larger lesion size and greater symptom severity. Solid lesions later formed cysts at a median 130 months. Growth in both total volume and solid component accelerated after cyst formation (10.6 and 6.0 times median rate prior to cyst emergence). VHL patients died at a younger age (47.9 vs 74.5, p = 0.0017) and were more likely to die of direct disease sequelae. Though treatment-free survival time was significantly longer in sporadic cases, a substantial fraction (> 40%) developed tumor recurrence/progression requiring additional treatment.
Conclusions
Hemangioblastoma presentation varies with etiology and clinical course is more complicated in VHL cases. Nodular lesions often develop cysts over time which is associated with accelerated tumor growth. Sporadic cases have a previously unappreciated but substantial risk of late recurrence/progression requiring treatment.




Similar content being viewed by others
References
Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P (1989) Hemangioblastomas of the central nervous system: a 10-year study with special reference to von Hippel–Lindau syndrome. J Neurosurg 70(1):24–30
Kano H, Shuto T, Iwai Y et al (2015) Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg 122(6):1469–1478
Hanakita S, Koga T, Shin M et al (2014) The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro Oncol 16(3):429–433
Lonser RR, Glenn GM, Walther M et al (2003) von Hippel–Lindau disease. The Lancet 361(9374):2059–2067
Iwai K, Yamanaka K, Kamura T et al (1999) Identification of the von Hippel–Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci USA 96(22):12436–12441
Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W (1999) The von Hippel–Lindau tumor suppressor protein is a component of an E3 ubiquitin–protein ligase activity. Genes Dev 13(14):1822–1833
Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Goldberg MA (1996) Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci USA 93(20):10595–10599
Maxwell PH, Wiesener MS, Chang G-W et al (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399(6733):271
Takayanagi S, Mukasa A, Tanaka S et al (2017) Differences in genetic and epigenetic alterations between von Hippel–Lindau disease-related and sporadic hemangioblastomas of the central nervous system. Neuro Oncol 19(9):1228–1236
Knudson AG (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68(4):820–823
Sato Y, Yoshizato T, Shiraishi Y et al (2013) Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 45(8):860
Maher E, Yates J, Harries R et al (1990) Clinical features and natural history of von Hippel–Lindau disease. QJM Int J Med 77(2):1151–1163
Nielsen SM, Rhodes L, Blanco IG et al (2016) Von Hippel–Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome 2016. Am Soc Clin Oncol. https://doi.org/10.1200/JCO.2015.65.6140
Lonser RR, Butman JA, Huntoon K et al (2014) Prospective natural history study of central nervous system hemangioblastomas in von Hippel–Lindau disease. J Neurosurg 120(5):1055–1062
Knudson AG Jr (1986) Genetics of human cancer. Annu Rev Genet 20(1):231–251
Knudson AG Jr, Strong LC (1972) Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet 24(5):514
Louis D, Ohgaki H, Wiestler O, Cavenee W (2016) WHO classification of tumours of the central nervous system (revised 4th edition). International Agency for Research on Cancer (IARC), Lyon
Pelliccia F, Limongelli G, Autore C, Gimeno-Blanes JR, Basso C, Elliott P (2019) Sex-related differences in cardiomyopathies. Int J Cardiol 286:239–243
Maher ER, Neumann HP, Richard S (2011) von Hippel–Lindau disease: a clinical and scientific review. Eur J Hum Genet 19(6):617–623
Woodward ER, Wall K, Forsyth J, Macdonald F, Maher ER (2007) VHL mutation analysis in patients with isolated central nervous system haemangioblastoma. Brain 130(3):836–842
Huntoon K, Wu T, Elder JB et al (2016) Biological and clinical impact of hemangioblastoma-associated peritumoral cysts in von Hippel–Lindau disease. J Neurosurg 124(4):971–976
Lonser RR, Vortmeyer AO, Butman JA et al (2005) Edema is a precursor to central nervous system peritumoral cyst formation. Ann Neurol 58(3):392–399
Acknowledgements
None.
Funding
Funding was provided by the Mayo Clinic Department of Neurological Surgery, Rochester, MN.
Author information
Authors and Affiliations
Contributions
Conceptualization: HT, IFP. Manuscript writing: HT. Final editing and approval of the manuscript: HT, CG, AP, DAB, FBM, TCB, IFP.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takami, H., Graffeo, C.S., Perry, A. et al. Presentation, imaging, patterns of care, growth, and outcome in sporadic and von Hippel–Lindau-associated central nervous system hemangioblastomas. J Neurooncol 159, 221–231 (2022). https://doi.org/10.1007/s11060-022-04021-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04021-8