Abstract
Purpose
Glioblastoma multiforme (GBM) is a primary brain tumor with devastating prognosis. Although the O6-methylguanine-DNA methyltransferase (MGMT) leads to inherent temozolomide (TMZ) resistance, approximately half of GBMs were sufficient to confer acquired TMZ resistance, which express low levels of MGMT. The purpose of this study was to investigate the underlying mechanisms of the acquired TMZ resistance in MGMT-deficient GBM.
Methods
The function of Down syndrome critical region protein 3 (DSCR3) on MGMT-deficient GBM was investigated in vitro and in an orthotopic brain tumor model in mice. Purification of plasma membrane proteins by membrane-cytoplasmic separation and subsequent label free-based quantitative proteomics were used to identified potential protein partners for DSCR3. Immunofluorescence was performed to show the reverse transport of solute carrier family 38 member 1 (SLC38A1) mediated by DSCR3.
Results
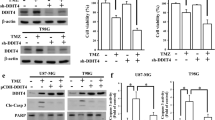
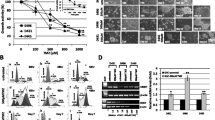
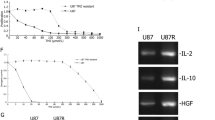
DSCR3 is upregulated in MGMT-deficient GBM cells during TMZ treatment. Both DSCR3 and SLC38A1 were highly expressed in recurrent GBM patients. Silencing DSCR3 or SLC38A1 expression can increase TMZ sensitivity in MGMT-deficient GBM cells. Combination of proteomics and in vitro experiments show that DSCR3 directly binds internalized SLC38A1 to mediate its sorting into recycling pathway, which maintains the abundance on plasma membrane and enhances uptake of glutamine in MGMT-deficient GBM cells.
Conclusions
DSCR3 is a crucial regulator of acquired TMZ resistance in MGMT-deficient GBM. The DSCR3-dependent recycling of SLC38A1 maintains its abundance on plasma membrane, leading to tumor progression and acquired TMZ resistance in MGMT-deficient GBM.





Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Alexander BM, Cloughesy TF (2017) Adult glioblastoma. J Clin Oncol 35(21):2402–2409
Tan AC et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312
Omuro A et al (2018) Multicenter phase IB trial of carboxyamidotriazole orotate and temozolomide for recurrent and newly diagnosed glioblastoma and other anaplastic gliomas. J Clin Oncol 36(17):1702–1709
Tomar MS et al (2021) Elucidating the mechanisms of temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer 1876(2):188616
Wick W et al (2014) MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol 10(7):372–385
Murphy SF et al (2016) Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res 76(1):139–149
Chen K et al (2019) Stimuli-responsive polymer-doxorubicin conjugate: antitumor mechanism and potential as nano-prodrug. Acta Biomater 84:339–355
Raote I, Malhotra V (2019) Protein transport by vesicles and tunnels. J Cell Biol 218(3):737–739
Foot N, Henshall T, Kumar S (2017) Ubiquitination and the regulation of membrane proteins. Physiol Rev 97(1):253–281
Guo J et al (2020) Quantitative proteomics analysis reveals nuclear perturbation in human glioma U87 cells treated with temozolomide. Cell Biochem Funct 38(2):185–194
McNally KE et al (2017) Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol 19(10):1214–1225
Chen KE, Healy MD, Collins BM (2019) Towards a molecular understanding of endosomal trafficking by retromer and retriever. Traffic 20(7):465–478
Yi GZ et al (2019) Acquired temozolomide resistance in MGMT-deficient glioblastoma cells is associated with regulation of DNA repair by DHC2. Brain 142(8):2352–2366
Broer A, Rahimi F, Broer S (2016) Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J Biol Chem 291(25):13194–13205
Zavorka Thomas ME et al (2021) Gilteritinib inhibits glutamine uptake and utilization in FLT3-ITD-positive AML. Mol Cancer Ther 20(11):2207–2217
Yuan R et al (2009) Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2:45
Murugan AK (2019) mTOR: role in cancer, metastasis and drug resistance. Semin Cancer Biol 59:92–111
Gremke N et al (2020) mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability. Nat Commun 11(1):4684
Nicklin P et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534
Jewell JL et al (2015) Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 347(6218):194–198
Weller M et al (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6(1):39–51
Yu W et al (2019) O(6)-methylguanine-DNA methyltransferase (MGMT): challenges and new opportunities in glioma chemotherapy. Front Oncol 9:1547
Doherty GJ, McMahon HT (2009) Mechanisms of endocytosis. Annu Rev Biochem 78:857–902
Ju Y et al (2020) Application of advances in endocytosis and membrane trafficking to drug delivery. Adv Drug Deliv Rev 157:118–141
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10(9):623–635
Claus LAN, Savatin DV, Russinova E (2018) The crossroads of receptor-mediated signaling and endocytosis in plants. J Integr Plant Biol 60(9):827–840
Smith SM, Renden R, von Gersdorff H (2008) Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci 31(11):559–568
Bhutia YD, Ganapathy V (2016) Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta 1863(10):2531–2539
Cha YJ, Kim ES, Koo JS (2018) Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci 19(3):907
Kandasamy P et al (2018) Amino acid transporters revisited: new views in health and disease. Trends Biochem Sci 43(10):752–789
Qureshi T et al (2019) The glutamine transporter Slc38a1 regulates GABAergic neurotransmission and synaptic plasticity. Cereb Cortex 29(12):5166–5179
Cruzat V et al (2018) Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10(11):1564
Yang L, Venneti S, Nagrath D (2017) Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng 19:163–194
Kuhn KS et al (2010) Glutamine as indispensable nutrient in oncology: experimental and clinical evidence. Eur J Nutr 49(4):197–210
Biswas SK (2015) Metabolic reprogramming of immune cells in cancer progression. Immunity 43(3):435–449
Teixeira E, Silva C, Martel F (2021) The role of the glutamine transporter ASCT2 in antineoplastic therapy. Cancer Chemother Pharmacol 87(4):447–464
Fu S et al (2019) Glutamine synthetase promotes radiation resistance via facilitating nucleotide metabolism and subsequent DNA damage repair. Cell Rep 28(5):1136-1143e4
Covarrubias AJ, Aksoylar HI, Horng T (2015) Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol 27(4):286–296
Jiang SH et al (2017) Increased serotonin signaling contributes to the Warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology 153(1):277-291e19
Martinez-Outschoorn UE et al (2017) Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14(1):11–31
Funding
This study was supported by the Natural Science Foundation of Guangdong Province, the National Natural Science Foundation of China (Grant Nos. 81803100), Key R&D Program of Guangdong Province (2018B090906001), Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University (2019J002) and Young innovative talents in Colleges and Universities in Guangdong Province (2021KQNCX022).
Author information
Authors and Affiliations
Contributions
RL was the major contributor in experiments performing and manuscript writing. YX was the major contributor in experiments performing. YL and S-TQ were the contributors of the design of the study. SX, YZ, BN, G-ZY and GH were the major contributors in data acquisition and specimen collection. SX, YZ, HW, BN, HS, ZW were contributors in experiments performing and analysis presentation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no competing interests.
Ethical approval
Ethics of animal and human research subjects has been reviewed by the Ethics Committee of Southern Medical University. Animal care complied with the Guide for the Care and Use of Laboratory Animals. For patient material, written consent will be sought from all participants prior to their inclusion in the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their anonymized data and photographs.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, R., Xu, Y., Xie, S. et al. Recycling of SLC38A1 to the plasma membrane by DSCR3 promotes acquired temozolomide resistance in glioblastoma. J Neurooncol 157, 15–26 (2022). https://doi.org/10.1007/s11060-022-03964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-03964-2