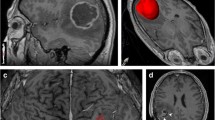
Abstract
Purpose
The peritumoral region (PTR) in glioblastoma (GBM) represents a combination of infiltrative tumor and vasogenic edema, which are indistinguishable on magnetic resonance imaging (MRI). We developed a radiomic signature by using imaging data from low grade glioma (LGG) (marker of tumor) and PTR of brain metastasis (BM) (marker of edema) and applied it on the GBM PTR to generate probabilistic maps.
Methods
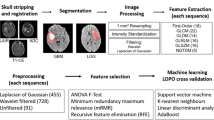
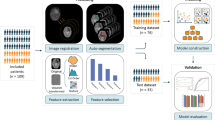
270 features were extracted from T1-weighted, T2-weighted, and apparent diffusion coefficient maps in over 3.5 million voxels of LGG (36 segments) and BM (45 segments) scanned in a 1.5T MRI. A support vector machine classifier was used to develop the radiomics model from approximately 50% voxels (downsampled to 10%) and validated with the remaining. The model was applied to over 575,000 voxels of the PTR of 10 patients with GBM to generate a quantitative map using Platt scaling (infiltrative tumor vs. edema).
Results
The radiomics model had an accuracy of 0.92 and 0.79 in the training and test set, respectively (LGG vs. BM). When extrapolated on the GBM PTR, 9 of 10 patients had a higher percentage of voxels with a tumor-like signature over radiological recurrence areas. In 7 of 10 patients, the areas under curves (AUC) were > 0.50 confirming a positive correlation. Including all the voxels from the GBM patients, the infiltration signature had an AUC of 0.61 to predict recurrence.
Conclusion
A radiomic signature can demarcate areas of microscopic tumors from edema in the PTR of GBM, which correlates with areas of future recurrence.
Graphic abstract




Similar content being viewed by others
Data availability
Data will be made available on request to the corresponding author following institutional ethics committee protocols.
Code availability
The radiomic feature extraction was performed using freely available Pyradiomics software (default set of features) (http://www.pyradiomics.io/pyradiomics.html). All standardization, model fitting, and assessment were performed using Scikit-Learn (https://scikit-learn.org/stable). The SVM classifier was used based on LIBSVM with a radial-basis-function kernel.
References
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446. https://doi.org/10.1016/j.ejca.2011.11.036
Villanueva-Meyer JE, Mabray MC, Cha S (2017) Current clinical brain tumor imaging. Neurosurgery 81:397–415. https://doi.org/10.1093/neuros/nyx103
Chaddad A, Kucharczyk MJ, Daniel P et al (2019) Radiomics in glioblastoma: current status and challenges facing clinical implementation. Front Oncol. https://doi.org/10.3389/fonc.2019.00374
Yamahara T, Numa Y, Oishi T et al (2010) Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol 27:81–87. https://doi.org/10.1007/s10014-010-0275-7
Barajas RF, Phillips JJ, Parvataneni R et al (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 14:942–954. https://doi.org/10.1093/neuonc/nos128
Eidel O, Burth S, Neumann J-O et al (2017) Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: a correlation with histopathology. PLoS ONE. https://doi.org/10.1371/journal.pone.0169292
Duron L, Balvay D, Perre SV et al (2019) Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS ONE. https://doi.org/10.1371/journal.pone.0213459
Carré A, Klausner G, Edjlali M et al (2020) Standardization of brain MR images across machines and protocols: bridging the gap for MRI-based radiomics. Sci Rep. https://doi.org/10.1038/s41598-020-69298-z
McDonald MW, Shu H-KG, Curran WJ, Crocker IR (2011) Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys 79:130–136. https://doi.org/10.1016/j.ijrobp.2009.10.048
Petrecca K, Guiot M-C, Panet-Raymond V, Souhami L (2013) Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol 111:19–23. https://doi.org/10.1007/s11060-012-0983-4
Lemée J-M, Clavreul A, Menei P (2015) Intratumoral heterogeneity in glioblastoma: don’t forget the peritumoral brain zone. Neuro Oncol 17:1322–1332. https://doi.org/10.1093/neuonc/nov119
Glas M, Rath BH, Simon M et al (2010) Residual tumor cells are unique cellular targets in glioblastoma. Ann Neurol 68:264–269. https://doi.org/10.1002/ana.22036
Ruiz-Ontañon P, Orgaz JL, Aldaz B et al (2013) Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells 31:1075–1085. https://doi.org/10.1002/stem.1349
Price SJ, Jena R, Burnet NG et al (2006) Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Yan J-L, Li C, Boonzaier NR et al (2019) Multimodal MRI characteristics of the glioblastoma infiltration beyond contrast enhancement. Ther Adv Neurol Disord 12:1756286419844664. https://doi.org/10.1177/1756286419844664
Blystad I, Warntjes JBM, Smedby Ö et al (2020) Quantitative MRI using relaxometry in malignant gliomas detects contrast enhancement in peritumoral oedema. Sci Rep 10:17986. https://doi.org/10.1038/s41598-020-75105-6
Akbari H, Macyszyn L, Da X et al (2016) Imaging surrogates of infiltration obtained via multiparametric imaging pattern analysis predict subsequent location of recurrence of glioblastoma. Neurosurgery 78:572–580. https://doi.org/10.1227/NEU.0000000000001202
Rathore S, Akbari H, Doshi J, et al (2018) Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: implications for personalized radiotherapy planning. JMI 5:021219. https://doi.org/10.1117/1.JMI.5.2.021219
Hu LS, Ning S, Eschbacher JM et al (2015) Multi-parametric MRI and texture analysis to visualize spatial histologic heterogeneity and tumor extent in glioblastoma. PLoS ONE 10:e0141506. https://doi.org/10.1371/journal.pone.0141506
Yan J-L, Li C, van der Hoorn A et al (2020) A neural network approach to identify the peritumoral invasive areas in glioblastoma patients by using MR radiomics. Sci Rep 10:9748. https://doi.org/10.1038/s41598-020-66691-6
Stewart J, Sahgal A, Lee Y et al (2020) Quantitating inter-fraction target dynamics during concurrent chemoradiation for glioblastoma: a prospective serial imaging study. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2020.10.002
Jackson C, Choi J, Khalafallah AM et al (2020) A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J Neurooncol 148:419–431. https://doi.org/10.1007/s11060-020-03556-y
Certo F, Altieri R, Maione M et al (2020) FLAIRectomy in supramarginal resection of glioblastoma correlates with clinical outcome and survival analysis: a prospective, single institution, Case Series. Oper Neurosurg (Hagerstown). https://doi.org/10.1093/ons/opaa293
Tseng C-L, Stewart J, Whitfield G et al (2020) Glioma consensus contouring recommendations from a MR-Linac International Consortium Research Group and evaluation of a CT-MRI and MRI-only workflow. J Neurooncol. https://doi.org/10.1007/s11060-020-03605-6
Kumar N, Kumar R, Sharma SC et al (2020) Impact of volume of irradiation on survival and quality of life in glioblastoma: a prospective, phase 2, randomized comparison of RTOG and MDACC protocols. Neurooncol Pract 7:86–93. https://doi.org/10.1093/nop/npz024
Azoulay M, Chang SD, Gibbs IC et al (2020) A phase I/II trial of 5-fraction stereotactic radiosurgery with 5-mm margins with concurrent temozolomide in newly diagnosed glioblastoma: primary outcomes. Neuro Oncol 22:1182–1189. https://doi.org/10.1093/neuonc/noaa019
Acknowledgements
We would like to thank the patients and their caregivers involved in the study. Our sincere gratitude to the Terry Fox Foundation Program Project Grant from the Hecht Foundation for the funding support associated with the study.
Funding
Terry Fox Foundation Program Project Grant from the Hecht Foundation (1083) awarded to Gregory J. Czarnota. The funding bodies had no influence on the study design, data collection, analysis, interpretation of data, or the manuscript's writing.
Author information
Authors and Affiliations
Contributions
Conceptualization: AD, BG, AS, GJC; Methodology: All authors; Formal Analysis and investigation: All authors; Writing-original draft preparation: AD, BG, AS, GJC; Writing-review and editing: All authors; Project administration and supervision: AS, GJC; Funding acquisition: GJC. All the authors are in agreement and accountable for all the aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Archya Dasgupta: None. Benjamin Geraghty: None. Pejman Maralani: None. Nauman Malik: None. Michael Sandhu: None. Jay Detsky: None. Chia-Lin Tseng: Travel accommodations/expenses & honoraria for past educational seminars by Elekta, belongs to the Elekta MR-Linac Research Consortium, and advisor/consultant with Sanofi. Hany Soliman: None. Sten Myrehaug: Travel accommodations/expenses from Elekta AB. Research support from Novartis/AAA. Zain Husain: Travel accommodations/expenses from Elekta. James Perry: None. Angus Lau: None. Arjun Sahgal: Advisor/consultant with AbbVie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board). Board Member: International Stereotactic Radiosurgery Society (ISRS). Past educational seminars with Elekta AB, Accuray Inc., Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon. Research grant with Elekta AB. Travel accommodations/expenses by Elekta, Varian, BrainLAB. Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. Gregory J. Czarnota: Funding received from the Terry Fox Foundation Program Project Grant.
Ethical approval
The study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre (Protocol Number: 034-2020).
Consent to participate
Consent was waived for the retrospective study.
Consent for publication
Not applicable (anonymized data, imaging study).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11060_2021_3762_MOESM2_ESM.pptx
Supplementary file 2—Supplementary Figure 1 shows the receiver operating characteristics curve in the test set of low grade glioma and peritumoral region of brain metastases for voxel-based classification (PPTX 112 kb)
Rights and permissions
About this article
Cite this article
Dasgupta, A., Geraghty, B., Maralani, P.J. et al. Quantitative mapping of individual voxels in the peritumoral region of IDH-wildtype glioblastoma to distinguish between tumor infiltration and edema. J Neurooncol 153, 251–261 (2021). https://doi.org/10.1007/s11060-021-03762-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03762-2