Abstract
Introduction
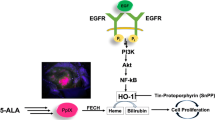
Although the utility 5-aminolevulinic acid (5-ALA)-mediated fluorescence-guided surgery (FGS) in meningiomas is increasingly discussed, data about the kinetics of protoporphyrin IX (PpIX) and tumor fluorescence are sparse.
Methods
PpIX kinetics after exposition to varying 5-ALA doses (12.5–150 µg/ml) was analyzed in two immortalized as well as primary WHO grade I and II meningioma and U87 high-grade glioma cell lines. Expression of FECH, ABCB6 and ABCG2 was investigated by quantitative real-time PCR.
Results
Fluorescence in Ben-Men 1 and primary WHO grade I/II meningioma increased with rising 5-ALA doses up to 100 µg/ml but then showed a saturation effect. However, decrease of fluorescence was slower after 150 than after 100 µg/ml 5-ALA. Fluorescence in U87 cells marginally increased with rising 5-ALA doses. Kinetics of the fluorescence in Ben-Men 1 cells did not differ from primary meningioma cells after 25–150 µg/ml 5-ALA (p > .05, each). No difference was found when comparing the fluorescence between primary grade I and II meningiomas after any 5-ALA dosage (p > .05, each). No relevant fluorescence was found in IOMM-Lee cells. Expression of FECH, ABCB6 and ABCG2 as well as PpIX export differed between all analyzed cell lines but were not connected to fluorescence.
Conclusions
Eligibility of established meningioma cell lines for in-vitro analyzes of tumor fluorescence significantly differs. Fluorescence in Ben-Men 1 and primary meningioma cell lines but less in IOMM Lee cells is 5-ALA dose-dependent, encouraging in-situ trials to encounter currently discussed shortcomings of FGS in meningiomas. Fluorescence is not related to expression of FECH, ABCB6 and ABCG2.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
No custom software code was written for data analysis in this study.
References
Ewelt C, Nemes A, Senner V, Wolfer J, Brokinkel B, Stummer W, Holling M (2015) Fluorescence in neurosurgery: Its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B 148:302–309. https://doi.org/10.1016/j.jphotobiol.2015.05.002
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
Foster N, Eljamel S (2016) ALA-induced fluorescence image guided surgery of meningiomas: a meta-analyses. Photodiagnosis Photodyn Ther 15:73–78. https://doi.org/10.1016/j.pdpdt.2016.05.006
Valdes PA, Millesi M, Widhalm G, Roberts DW (2019) 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J Neurooncol 141:555–565. https://doi.org/10.1007/s11060-018-03079-7
Gousias K, Schramm J, Simon M (2016) The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg 125:551–560. https://doi.org/10.3171/2015.9.JNS15754
Spille DC, Hess K, Bormann E, Sauerland C, Brokinkel C, Warneke N, Mawrin C, Paulus W, Stummer W, Brokinkel B (2020) Risk of tumor recurrence in intracranial meningiomas: comparative analyses of the predictive value of the postoperative tumor volume and the Simpson classification. J Neurosurg. https://doi.org/10.3171/2020.4.JNS20412
Cornelius JF, Slotty PJ, El Khatib M, Giannakis A, Senger B, Steiger HJ (2014) Enhancing the effect of 5-aminolevulinic acid based photodynamic therapy in human meningioma cells. Photodiagnosis Photodyn Ther 11:1–6. https://doi.org/10.1016/j.pdpdt.2014.01.001
El-Khatib M, Tepe C, Senger B, Dibue-Adjei M, Riemenschneider MJ, Stummer W, Steiger HJ, Cornelius JF (2015) Aminolevulinic acid-mediated photodynamic therapy of human meningioma: an in vitro study on primary cell lines. Int J Mol Sci 16:9936–9948. https://doi.org/10.3390/ijms16059936
Kaneko S, Brokinkel B, Suero Molina E, Warneke N, Holling M, Bunk EC, Hess K, Senner V, Paulus W, Stummer W (2020) Real-time in vivo kinetics of protoporphyrin IX after administration of 5-aminolevulinic acid in meningiomas and comparative analyses with glioblastomas. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-020-04353-2
Puttmann S, Senner V, Braune S, Hillmann B, Exeler R, Rickert CH, Paulus W (2005) Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest 85:1163–1171. https://doi.org/10.1038/labinvest.3700307
Lee WH (1990) Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery 27:389–395; discussion 396
Hardy SJ, Christodoulides M, Weller RO, Heckels JE (2000) Interactions of Neisseria meningitidis with cells of the human meninges. Mol Microbiol 36:817–829. https://doi.org/10.1046/j.1365-2958.2000.01923.x
Sun W, Kajimoto Y, Inoue H, Miyatake S, Ishikawa T, Kuroiwa T (2013) Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn Ther 10:42–50. https://doi.org/10.1016/j.pdpdt.2012.06.003
Millesi M, Kiesel B, Mischkulnig M, Martinez-Moreno M, Wohrer A, Wolfsberger S, Knosp E, Widhalm G (2016) Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J Neurosurg 125:1408–1419. https://doi.org/10.3171/2015.12.JNS151513
Potapov AA, Goryaynov SA, Danilov GV, Chelushkin DM, Okhlopkov VA, Shimanskiy VN, Beshplav ST, Poshataev VK, Shishkina LV, Zakharova NE, Spallone A, Savel’eva TA, Loshchenov VB (2018) [Intraoperative fluorescence diagnostics in surgery of intracranial meningiomas: analysis of 101 cases]. Zh Vopr Neirokhir Im N N Burdenko 82:17–29. https://doi.org/10.17116/oftalma201882217-29
Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H (2010) Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir (Wien) 152:1711–1719. https://doi.org/10.1007/s00701-010-0708-4
Brokinkel B, Kroger S, Senner V, Jeibmann A, Karst U, Stummer W (2018) Visualizing protoporphyrin IX formation in the dura tail of meningiomas by mass spectrometry imaging. Acta Neurochir (Wien) 160:1433–1437. https://doi.org/10.1007/s00701-018-3488-x
Knipps J, Beseoglu K, Kamp M, Fischer I, Felsberg J, Neumann LM, Steiger HJ, Cornelius JF (2017) Fluorescence behavior and dural infiltration of meningioma analyzed by 5-aminolevulinic acid-based fluorescence: operating microscope versus mini-spectrometer. World Neurosurg 108:118–127. https://doi.org/10.1016/j.wneu.2017.08.140
Turcotte EL, Rahme RJ, Merrill SA, Hess RA, Lettieri SC, Bendok BR (2020) Three applications of 5-aminolevulinic acid (5-ALA) for microsurgical resection of meningiomas. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.03.178
Teng L, Nakada M, Zhao SG, Endo Y, Furuyama N, Nambu E, Pyko IV, Hayashi Y, Hamada JI (2011) Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 104:798–807. https://doi.org/10.1038/bjc.2011.12
Zhao SG, Chen XF, Wang LG, Yang G, Han DY, Teng L, Yang MC, Wang DY, Shi C, Liu YH, Zheng BJ, Shi CB, Gao X, Rainov NG (2013) Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol 20:4379–4388. https://doi.org/10.1245/s10434-011-2201-6
Hefti M, Holenstein F, Albert I, Looser H, Luginbuehl V (2011) Susceptibility to 5-aminolevulinic acid based photodynamic therapy in WHO I meningioma cells corresponds to ferrochelatase activity. Photochem Photobiol 87:235–241. https://doi.org/10.1111/j.1751-1097.2010.00821.x
Valdes PA, Bekelis K, Harris BT, Wilson BC, Leblond F, Kim A, Simmons NE, Erkmen K, Paulsen KD, Roberts DW (2014) 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurgery 10 Suppl 1:74–82; discussion 82 – 73. https://doi.org/10.1227/NEU.0000000000000117
Acknowledgements
We cordially thank Hans-Joachim Schnittler, Institute of Anatomy and Vascular Biology, University Hospital Muenster, Muenster, Germany, for kindly providing the necessary access to the microplate reader. We further thank Christian Mawrin, Institute of Neuropathology, Magdeburg, Germany, for kindly providing IOMM-Lee Meningioma cell lines.
Funding
Partial financial support was received from the Walter Schulz Stiftung, Germany.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ECB, BB and VS. The first draft of the manuscript was written by BB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local ethics committee and permitted by the patients in each single case (2019-296-f-S).
Consent to participate/for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Suppl. Figure 1: Phase contrast microscopy of primary meningioma cells.
Cells could adhere to different coatings including Matrigel (A), PLL plus mLaminin (B) and PLL plus hLaminin (C) as well as to culture plates without any coating and could be kept over several passages. Cells could also be kept in culture for several passages following kryoconservation (D) (div.=days in vitro, h = human, m = mouse, PLL = poly-L-Lysine) (A – C: WHO grade II, atypical meningioma, MIB1 Proliferation-index ND; D: WHO grade I, meningothelial meningioma, MIB1 Proliferation-index 5%). (TIF 17932 kb)
Suppl. Figure 2: Fluorescence intensity in negative controls
. As expected, no relevant fluorescence was detected in negative controls. (TIF 7922 kb)
Suppl. Figure 3: Fluorescence of primary and immortalized meningioma cell lines in comparative analyses.
Fluorescence intensity of Ben-Men 1 cells at 0, 2, 4 and 6 hours was similar as compared to primary meningioma cells after 25–150 µg/ml 5-ALA (p > .05, each). On the other hand, fluorescence intensity between primary WHO grade II meningioma cell lines and IOMM-Lee cells began to differ at 0 and 2 hours after incubation (p = .025 and 0.053) with 25 µg/ml 5-ALA. At 50 µg/ml 5-ALA, fluorescence intensity between both cell lines significantly differed at all time points (p = .036) with a similar trend at doses of 100 and 150 mg/ml 5-ALA (p = .083, each). For better overview exemplary data shown only for 50 µg/ml 5-ALA. (TIF 7523 kb)
Rights and permissions
About this article
Cite this article
Bunk, E.C., Wagner, A., Stummer, W. et al. 5-ALA kinetics in meningiomas: analysis of tumor fluorescence and PpIX metabolism in vitro and comparative analyses with high-grade gliomas. J Neurooncol 152, 37–46 (2021). https://doi.org/10.1007/s11060-020-03680-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03680-9