Abstract
Purpose
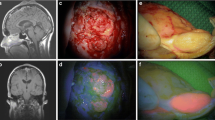
Stereotactic biopsies are routinely used to establish a histological diagnosis of unclear cerebral pathologies. Intraoperatively, frozen-section analysis often confirms diagnostic tissue but also exhibits methodological pitfalls. Intraoperative five-aminolevulinic acid (5-ALA)-fluorescence has been described not only in gliomas but also in other cerebral pathologies. In this study, we assessed the 5-ALA contribution to the intraoperative confirmation of diagnostic tissue in frame-based stereotactic biopsies of unclear intracerebral lesions in direct comparison with frozen-section analysis.
Methods
Patients scheduled for stereotactic biopsies of unclear intracerebral pathologies received 5-ALA preoperatively. Obtained samples were intraoperatively analyzed for the presence of 5-ALA-fluorescence. One sample was used for frozen-section and a second one for permanent histopathological analysis. The diagnostic yield of frozen-section and intraoperative 5-ALA-fluorescence was calculated. The inclusion criteria for this retrospective analysis were unclear intracerebral lesions with inconclusive imaging findings and several differential diagnoses.
Results
A total of 39 patients with 122 obtained specimens were included. The overall diagnostic yield was 92.3%. 5-ALA-positive samples were obtained in 74.3% (29/39) of patients and all these samples contained diagnostic tissue. 5-ALA-fluorescence confirmed diagnostic tissue with a sensitivity of 100%, a specificity of 27%, a positive predictive value (PPV) of 78%, and a negative predictive value (NPV) of 100%. A clear diagnosis could be predicted by frozen section with a sensitivity of 80%, a specificity of 100%, a PPV of 100%, and NPV of 30%; Fisher’s exact test p = 0.01.
Conclusion
The 5-ALA-fluorescence in stereotactic biopsies of unclear intracerebral pathologies exhibits a high PPV/NPV for intraoperative confirmation of diagnostic tissue and might increase the diagnostic yield of the procedure by overcoming some of the limitations of frozen-section.


Similar content being viewed by others
Availability of data and material
All available data is presented in the manuscript.
References
Livermore LJ, Ma R, Bojanic S, Pereira EA (2014) Yield and complications of frame-based and frameless stereotactic brain biopsy—the value of intraoperative histological analysis. Br J Neurosurg 28:637–644
Owen CM, Linskey ME (2009) Frame-based stereotaxy in a frameless era: current capabilities, relative role, and the positive- and negative predictive values of blood through the needle. J Neurooncol 93:139–149
Kiesel B, Millesi M, Woehrer A, Furtner J, Bavand A, Roetzer T, Mischkulnig M, Wolfsberger S, Preusser M, Knosp E, Widhalm G (2018) 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: experience in 41 patients. Neurosurg Focus 44:1–8
Von Campe G, Moschopulos M, Hefti M (2012) 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence as immediate intraoperative indicator to improve the savety of malignant or high-grade brain tumor diagnosis in frameless stereotactic biopsies. Acta Neurochir 154:585–588
Amraei R, Moradi A, Zham H, Ahadi M, Baikpour M, Rakhshan A (2017) A comparison between the diagnostic accuracy of frozen section and permanent section analysis in central nervous system. Asian Pac J Cancer Prev 18:659–666
Chand P, Amit S, Gupta R, Agarwal A (2016) Errors, limitations, and pitfalls in the diagnosis of central and peripheral nervous system lesions in intraoperative cytology and frozen sections. J Cytol 33:93–97
Shakal AA, Mokbel EA (2014) Hemorrhage after stereotactic biopsy from intra-axial brain lesions: incidence and avoidance. J Neurol Surg A Cent Eur Neurosurg 75:177–182
Grossmann R, Sadetzki S, Spiegelmann R, Ram Z (2005) Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir 147:627–631
Quick-Weller J, Tichy J, Harter PN, Tritt S, Baumgarten P, Bähr O, Dinc N, Behmanesh B, Weise L, Seifert V, Marquardt G (2018) “Two is not enough”—impact of the number of tissue samples obtained from stereotactic brain biopsies in suspected glioblastoma. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2017.09.032
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-Glioma study group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomized controlled multicenter phase III trial. Lancet Oncol 7:392–401
Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the surgical benefit of utilizing 5-ALA for fluorescence-guided surgery of malignant gliomas. Neurosurgery 77:663–673
Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi AR, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36:1–10
Schatlo B, Stockhammer F, Barrantes-Freer A, Bleckmann A, Siam L, Pukrop T, Rohde V (2019) 5-Aminolevulinic acid fluorescence indicates perilesional brain infiltration in brain metastases. World Neurosurg 5:100069
Raabe A, Krishnan R, Zimmermann M, Seifert V (2003) Frame-less and frame-based stereotaxy? How to choose the appropriate procedure. Cent Eur J Neurosurg 64:1–5
Smith JS, Quinones-Hinojosa A, Barbaro NM, McDermott MW (2005) Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol 73:173–179
Lefranc M, Capel C, Pruvot-Occean AS, Fichten A, Desenclos C, Toussaint P, Le Gars D, Peltier J (2015) Frameless robotic stereotactic biopsies: a consecutive series of 100 cases. J Neurosurg 122:342–352
Yasin H, Hoff HJ, Blümcke I, Simon M (2019) Experience with 102 frameless stereotactic biopsies using the neuromata robotic device. World Neurosurg 123:e450–e456
Terrier L, Gilard V, Marguet F, Fontanilles M, Derrey S (2019) Stereotactic brain biopsy: evaluation of robot-assisted procedure in 60 patients. Acta Neurochir 161:545–552
Lara-Almunia M, Hernandez-Vicente J (2019) Frame-based stereotactic biopsy: description and association of anatomical, radiologic, and surgical variables with diagnostic yield in a series of 407 cases. J Neurol Surg A Cent Eur Neurosurg 80:149–161
Hamisch CA, Minartz J, Blau T, Hafkemeyer V, Rueß D, Hellerbach A, Grau SJ, Ruge MI (2019) Frame-based stereotactic biopsy of deep-seated and midline structures in 511 procedures: feasibility, risk profile, and diagnostic yield. Acta Neurochir 161:2065–2071
Dhawan S, He Y, Bartek J Jr, Alattar AA, Chen CC (2019) Comparison of frame-based versus frameless intracranial stereotactic biopsy: systematic review and meta-analysis. World Neurosurg 127:607–616
Treglia G, Muoio B, Trevisi G, Mattoli MV, Albano D, Bertagna F, Giovanella L (2019) Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: a systematic review of published meta-analysis. Int J Mol Sci 20:4669
Widhalm G, Kiesel B, Woehrer A, Traub-Weidinger T, Preusser M, Marosi C, Prayer D, Hainfellner JA, Knosp E, Wolfsberger S (2013) 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 18:e76988
Catapano G, Sgulo FG, Seneca V, Iorio G, de Notaris M, di Nuzzo G (2018) Fluorescein-assisted stereotactic needle biopsy of brain tumors: a single-center experience and systematic review. Neurosurg Rev. https://doi.org/10.1007/s10143-018-0947-z
Marhold F, Mercea PA, Schleichel F, Berghoff AS, Heicappell P, Kiesel B, Mischkulnig M, Borkovec M, Wolfsberger S, Woehrer A, Preusser M, Knosp E, Ungersboeck K, Widhalm G (2019) Detailed analysis of 5-aminolevulinic acid induced fluorescence in different brain metastases at two specialized neurosurgical centers: experience in 157 cases. J Neurosurg 27:1–12
Goryaynov SA, Okhopkov VA, Golbin DA, Chernyshov KA, Svistov DV, Martynov BV, Kim AV, Byvaltsev VA, Pavlova GV, Batalov A, Konovalov NA, Zelenkov PV, Loschenov VB, Potapov AA (2019) Fluorescence diagnosis in neurooncology: retrospective analysis of 653 cases. Front Oncol 9:830
Millesi M, Kiesel B, Wöhrer A, Mercea PA, Bissolo M, Roertzer T, Wolfsberger S, Furtner J, Knosp E, Widhalm G (2019) Is intraoperative pathology needed if 5-aminolevulinic acid induced tissue fluorescence is found in stereotactic brain tumor biopsy? Neurosurgery. https://doi.org/10.1093/neuros/nyz086
Kamp MA, Felsberg J, Sadat H, Kuzibaev J, Steiger HJ, Rapp M, Reifenberger G, Dibue M, Sabel M (2015) 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir 157:207–213
Behling F, Hennersdorf F, Bornemann A, Tatagiba M, Skardelly M (2016) 5-Aminolevulinic acid accumulation in a cerebral infarction mimicking high grade glioma. World Neurosurg 91:586
Steinmann J, Rapp M, Turowski B, Steiger HJ, Cornelius JF, Sabel M, Kamp MA (2018) 5-ALA fluorescence behavior of cerebral infectious and inflammatory disease. Neurosurg Rev 41:365–369
Ji SY, Kim JW, Park CK (2019) Experience profiling of fluorescence-guided surgery II: non-glioma pathologies. Brain Tumor Res Treat 7:105–111
Funding
The study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
Due to retrospective nature of the study informed consent was waived.
Ethical approval
The study was approved by the local ethics committee of the University Medicine Göttingen (Study Identification Number 16/9/18). Due to retrospective nature of the study informed consent was deemed not necessary and all the procedures being performed were part of the routine care. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malinova, V., von Eckardstein, K., Mielke, D. et al. Diagnostic yield of fluorescence-assisted frame-based stereotactic biopsies of intracerebral lesions in comparison with frozen-section analysis. J Neurooncol 149, 315–323 (2020). https://doi.org/10.1007/s11060-020-03608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03608-3