Abstract
Purpose
Awake surgery is an established technique for resection of low-grade gliomas, while its possible benefit for resection of high-grade gliomas (HGGs) needs further confirmations. This retrospective study aims to compare overall survival, extent of resection (EOR) and cognitive outcome in two groups of HGGs patients submitted to asleep or awake surgery.
Methods
Thirty-three patients submitted to Gross Total Resection of contrast-enhancing area of HGGs were divided in two homogeneous groups: awake (AWg; N = 16) and asleep surgery (ASg; N = 17). All patients underwent to an extensive neuropsychological assessment before surgery (time_1), 1-week (time_2) and 4-months (time_3) after surgery. We performed analyses to assess differences in cognitive performances between groups, cognitive outcomes in each group and EOR. A comparison of overall survival (OS) between the two groups was conducted.
Results
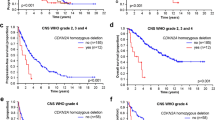
Statistical analyses showed no differences between groups at time_2 and time_3 in each cognitive domain, excluding selective attention that resulted higher in the AWg before surgery. Regarding cognitive outcomes, we found a reversible worsening of memory and constructional praxis, and a significant recovery at time_3, similar for both groups. Assessment of time_3 in respect to time_1 never showed differences (all ps > .074). Moreover we found a significant lower level of tumor infiltration after surgery for AWg (p < .05), with an influence on OS (p < .05). Indeed, patients of AWg showed a significant longer OS in comparison to those in the ASg (p < .01). This result was confirmed even considering only wildtype Glioblastoma (p < .05).
Conclusion
These results indicate that awake surgery, and in general a supra-total resection of enhancing area, can improve OS in HGGs patients, preserving neuro-cognitive profile and quality of life.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
References
Stupp R, Brada M, van den Bent MJ et al (2014) High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii93–iii101. https://doi.org/10.1093/annonc/mdu050
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Buckner JC (2003) Factors influencing survival in high-grade gliomas. Semin Oncol 30:10–14. https://doi.org/10.1053/J.SEMINONCOL.2003.11.031
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Hervey-Jumper SL, Berger MS (2014) Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol 16:284. https://doi.org/10.1007/s11940-014-0284-7
Dallabona M, Sarubbo S, Merler S et al (2017) Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neuro-Oncology Pract. https://doi.org/10.1093/nop/npw030
De Witt Hamer PC, Robles SG, Zwinderman AH et al (2012) Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565. https://doi.org/10.1200/JCO.2011.38.4818
Duffau H, Lopes M, Arthuis F et al (2005) Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851. https://doi.org/10.1136/jnnp.2004.048520
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764
Capelle L, Fontaine D, Mandonnet E et al (2013) Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg. https://doi.org/10.3171/2013.1.JNS121
Kotrotsou A, Elakkad A, Sun J et al (2018) Multi-center study finds postoperative residual non-enhancing component of glioblastoma as a new determinant of patient outcome. J Neurooncol. https://doi.org/10.1007/s11060-018-2850-4
Papagno C, Casarotti A, Comi A et al (2012) Measuring clinical outcomes in neuro-oncology. A battery to evaluate low-grade gliomas (LGG). J Neurooncol 108:269–275. https://doi.org/10.1007/s11060-012-0824-5
Taphoorn MJ, Klein M (2004) Cognitive deficits in adult patients with brain tumours. Lancet Neurol 3:159–168. https://doi.org/10.1016/S1474-4422(04)00680-5
Duffau H (2012) Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien) 154:575–584. https://doi.org/10.1007/s00701-011-1216-x
Klein M, Duffau H, De Witt Hamer PC (2012) Cognition and resective surgery for diffuse infiltrative glioma: An overview. J Neurooncol 108:309–318. https://doi.org/10.1007/s11060-012-0811-x
Mandonnet E, De Witt HP, Poisson I et al (2015) Initial experience using awake surgery for glioma: oncological, functional, and employment outcomes in a consecutive series of 25 cases. Neurosurgery 76:382–389. https://doi.org/10.1227/NEU.0000000000000644
Mandonnet E, Sarubbo S, Duffau H (2017) Proposal of an optimized strategy for intraoperative testing of speech and language during awake mapping. Neurosurg Rev 40:29–35. https://doi.org/10.1007/s10143-016-0723-x
Sarubbo S, Latini F, Panajia A et al (2011) Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurol Sci. https://doi.org/10.1007/s10072-011-0587-3
Sarubbo S, Latini F, Sette E et al (2012) Is the resection of gliomas in Wernicke’s area reliable? Acta Neurochir (Wien) 154:1653–1662. https://doi.org/10.1007/s00701-012-1416-z
Gerritsen JKW, Viëtor CL, Rizopoulos D et al (2019) Awake craniotomy versus craniotomy under general anesthesia without surgery adjuncts for supratentorial glioblastoma in eloquent areas: a retrospective matched case-control study. Acta Neurochir (Wien) 161:307–315. https://doi.org/10.1007/s00701-018-03788-y
Molinaro AM, Hervey-Jumper S, Morshed RA et al (2020) Association of maximal extent of resection of contrast-enhanced and non–contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 6(4):495–503. https://doi.org/10.1001/jamaoncol.2019.6143
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Nossek E, Matot I, Shahar T et al (2013) Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor; Clinical article. J Neurosurg 118:243–249
Santini B, Talacchi A, Casagrande F et al (2012) Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: a pilot study. J Neurosurg Anesthesiol 24:209–216
Brown T, Shah AH, Bregy A et al (2013) Awake craniotomy for brain tumor resection: the rule rather than the exception? J Neurosurg Anesthesiol 25:240–247
Von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577
Laiacona M, Barbarotto R, Trivelli C, Capitani E (1993) Dissociazioni semantiche intercategoriali: descrizione di una batteria standardizzata e dati normativi. Arch Psicol Neurol Psichiatr
Novelli G, Papagno C, Capitani E et al (1986) Tre test clinic di ricerca e produzione lessicale. Taratura su soggetti normali. Arch di Psicol Neurol e Psichiatr 4:477–506
Orsini A, Grossi D, Capitani E et al (1987) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8:537–548. https://doi.org/10.1007/BF02333660
Carlesimo GA, Caltagirone C, Gainotti G et al (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36:378–384. https://doi.org/10.1159/000117297
Caffarra P, Vezzadini G, Dieci F et al (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447. https://doi.org/10.1007/s100720200003
Spinnler H, Tognoni G (1987) Taratura e standardizzazione italiana di test neuropsicologici. Ital J Neurol Sci 8:8–120
Gainotti G, Marra C, Villa G (2001) A double dissociation between accuracy and time of execution on attentional tasks in Alzheimer’s disease and multi-infarct dementia. Brain 124:731–738. https://doi.org/10.1093/brain/124.4.731
Giovagnoli AR, Del Pesce M, Mascheroni S et al (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17:305–309
Robinson G, Shallice T, Bozzali M, Cipolotti L (2012) The differing roles of the frontal cortex in fluency tests. Brain 135:2202–2214. https://doi.org/10.1093/brain/aws142
Sarubbo S, Tate M, De Benedictis A et al (2020) Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205:116237. https://doi.org/10.1016/j.neuroimage.2019.116237
Sarubbo S, Tate M, De Benedictis A et al (2020) A normalized dataset of 1821 cortical and subcortical functional responses collected during direct electrical stimulation in patients undergoing awake brain surgery. Data Br. https://doi.org/10.1016/j.dib.2019.104892
Zacà D, Corsini F, Rozzanigo U et al (2018) Whole-brain network connectivity underlying the human speech articulation as emerged integrating direct electric stimulation, resting state fMRI and tractography. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2018.00405
Zacà D, Jovicich J, Corsini F et al (2018) ReStNeuMap: a tool for automatic extraction of resting-state functional MRI networks in neurosurgical practice. J Neurosurg JNS. https://doi.org/10.3171/2018.4.JNS18474
Sarubbo S, De Benedictis A, Merler S et al (2016) Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37:3858–3872. https://doi.org/10.1002/hbm.23281
Coello AF, Moritz-Gasser S, Martino J et al (2013) Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg. https://doi.org/10.3171/2013.6.JNS122470
Sarubbo S, De Benedictis A, Merler S et al (2015) Towards a functional atlas of human white matter. Hum Brain Mapp 36:3117–3136
Duffau H, Capelle L, Denvil D et al (2003) Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry 74:901–907. https://doi.org/10.1136/JNNP.74.7.901
Sarubbo S, De Benedictis A, Milani P et al (2015) The course and the anatomo-functional relationships of the optic radiation: A combined study with “post mortem” dissections and “in vivo” direct electrical mapping. J Anat. https://doi.org/10.1111/joa.12254
Herbet G, Lafargue G, Bonnetblanc F et al (2014) Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain. https://doi.org/10.1093/brain/awt370
Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF (2000) Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 19:1059–1079
Austin PC, Steyerberg EW (2015) The number of subjects per variable required in linear regression analyses. J Clin Epidemiol 68:627–636
Riley RD, Snell KI, Ensor J et al (2019) Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med 38:1276–1296
Schupper AJ, Hirshman BR, Carroll KT et al (2017) Effect of gross total resection in world health organization grade II Astrocytomas: SEER-based survival analysis. World Neurosurg 103:741–747. https://doi.org/10.1016/J.WNEU.2017.03.140
Mitchell AJ, Kemp S, Benito-León J, Reuber M (2010) The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatr 22:2–13. https://doi.org/10.1111/j.1601-5215.2009.00439.x
Douw L, Klein M, Fagel SS et al (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8:810–818. https://doi.org/10.1016/S1474-4422(09)70204-2
Acknowledgements
The authors would express their gratitude to the entire nurses teams of the Division of Neurosurgery and Anesthesiology for the daily interest, diligence and support, and the Direction Team of the APSS for their support to the work of the Division of Neurosurgery in the neuro-oncology field.
Funding
The extensive neuropsychological analyses of the NePsi Project (Division of Neurosurgery, “Santa Chiara Hospital”, Trento, Italy) included in this paper were supported by CARITRO Foundation (Trento, Italy) and the Direction Team of the Azienda Provinciale per I Servizi Sanitari of Trento.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This retrospective study respects the ethical standards of the Declaration of Helsinki (BMJ 1991; 302: 1194).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2020_3494_MOESM8_ESM.tif
Online Resource Figure 1: Cumulative hazard for patients with post-operative tumor infiltration index < or > 9. Supplementary file8 (TIF 370 kb)
11060_2020_3494_MOESM9_ESM.tif
Online Resource Figure 2: Kaplan-Meier curves for patients with post-operative tumor infiltration index < or > 9. Supplementary file9 (TIF 419 kb)
Rights and permissions
About this article
Cite this article
Zigiotto, L., Annicchiarico, L., Corsini, F. et al. Effects of supra-total resection in neurocognitive and oncological outcome of high-grade gliomas comparing asleep and awake surgery. J Neurooncol 148, 97–108 (2020). https://doi.org/10.1007/s11060-020-03494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03494-9