Abstract
Purpose
Dysembryoplastic neuroepithelial tumors (DNETs) are a common cause of chronic drug-resistant epilepsy and are known for their favorable surgical outcomes. Nevertheless, the seizure recurrence-free rate is not as favorable if tumorous nodules are present near the main mass. We call these small tumorous nodules in the vicinity of the main mass satellite lesions (SLs). We analyzed tumor and seizure control in the presence and following the subsequent removal of SLs.
Methods
We retrospectively reviewed the medical records, radiological data, and surgical procedures to obtain the outcomes of children who underwent resection surgery for DNET. The analyses were designed to address the associations among the demographic, tumor and seizure-related variables. A Cox proportional hazard model was used for the univariate and multivariate analyses.
Results
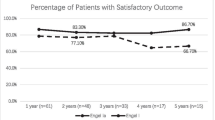
In total, 39 consecutive patients were included (26 males and 13 females). SLs were found in 22 patients (56%). The year-to-year analysis of patients with Engel class I was approximately 80% during the follow-up period. However, the actual seizure recurrence-free survival (RFS) rate was 82, 73 and 70% at the first, second and fifth year, respectively. The patients who initially presented with SLs had 46% seizure recurrence rates, while those without SL had 18% seizure recurrence rates.
Conclusions
As the seizure-RFS rate significantly declines over time, a more accurate seizure-free rate analysis using survival curves could be important for determining the outcome of DNET surgery. A thorough review identifying satellite lesions preoperatively and using intraoperative neuronavigation, electrocorticography (ECoG) or intraoperative ultrasonography is warranted to accomplish the wide resection of tumors with accompanying satellite lesions.



Similar content being viewed by others
Data availability
The datasets of the current study are available from the corresponding author on request.
References
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfafflin M, Elger C, Widman G, Schramm J, Becker A, Braun KP, Leijten F, Baayen JC, Aronica E, Chassoux F, Hamer H, Stefan H, Rossler K, Thom M, Walker MC, Sisodiya SM, Duncan JS, McEvoy AW, Pieper T, Holthausen H, Kudernatsch M, Meencke HJ, Kahane P, Schulze-Bonhage A, Zentner J, Heiland DH, Urbach H, Steinhoff BJ, Bast T, Tassi L, Lo Russo G, Ozkara C, Oz B, Krsek P, Vogelgesang S, Runge U, Lerche H, Weber Y, Honavar M, Pimentel J, Arzimanoglou A, Ulate-Campos A, Noachtar S, Hartl E, Schijns O, Guerrini R, Barba C, Jacques TS, Cross JH, Feucht M, Muhlebner A, Grunwald T, Trinka E, Winkler PA, Gil-Nagel A, Toledano Delgado R, Mayer T, Lutz M, Zountsas B, Garganis K, Rosenow F, Hermsen A, von Oertzen TJ, Diepgen TL, Avanzini G, Consortium E (2017) Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med 377(17):1648–1656. https://doi.org/10.1056/nejmoa1703784
Luyken C, Blumcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, Schramm J (2003) The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia 44(6):822–830
Fried I, Kim JH, Spencer DD (1994) Limbic and neocortical gliomas associated with intractable seizures: a distinct clinicopathological group. Neurosurgery 34(5):815–824
Maria T, Ingmar B, Eleonora A (2012) Long-term epilepsy-associated tumors. Brain Pathol 22(3):350–379. https://doi.org/10.1111/j.1750-3639.2012.00582.x
Daumas-Duport C, Scheithauer BW, Chodkiewicz J-P, Laws JER, Vedrenne C (1988) Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizuresreport of thirty-nine cases. Neurosurgery 23(5):545–556. https://doi.org/10.1227/00006123-198811000-00002
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Bonney PA, Boettcher LB, Conner AK, Glenn CA, Briggs RG, Santucci JA, Bellew MR, Battiste JD, Sughrue ME (2016) Review of seizure outcomes after surgical resection of dysembryoplastic neuroepithelial tumors. J Neurooncol 126(1):1–10. https://doi.org/10.1007/s11060-015-1961-4
Nguyen HS, Doan N, Gelsomino M, Shabani S (2017) Dysembryoplastic neuroectodermal tumor: an analysis from the Surveillance, Epidemiology, and End Results Program, 2004–2013. World Neurosurgery 103:380–385. https://doi.org/10.1016/j.wneu.2017.04.093
Zhang J-g Hu, W-z Zhao R-j, L-f Kong (2014) Dysembryoplastic neuroepithelial tumor: a clinical, neuroradiological, and pathological study of 15 cases. J Child Neurol 29(11):1441–1447. https://doi.org/10.1177/0883073813490831
Santos MV, de Oliveira RS, Machado HR (2014) Approach to cortical dysplasia associated with glial and glioneuronal tumors (FCD type IIIb). Child’s Nervous System 30(11):1869–1874. https://doi.org/10.1007/s00381-014-2519-z
Daghistani R, Miller E, Kulkarni AV, Widjaja E (2013) Atypical characteristics and behavior of dysembryoplastic neuroepithelial tumors. Neuroradiology 55(2):217–224. https://doi.org/10.1007/s00234-013-1135-z
Maher CO, White JB, Scheithauer BW, Raffel C (2008) Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg 44(4):333–336
Yasargil MG (1984) Microneurosurgery: operative treatment of CNS tumors 4A. Thieme Stratton, New York
Kim YH, Kim JS, Lee SK, Chung CK (2017) Neurologic outcome after resection of parietal lobe including primary somatosensory cortex: implications of additional resection of posterior parietal cortex. World Neurosurg 106:884–890. https://doi.org/10.1016/j.wneu.2017.07.066
Engel J Jr (1993) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies. Raven Press, New York, pp 609–621
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE commission on therapeutic strategies. Epilepsia 51(6):1069–1077
Sampetrean O, Maehara T, Arai N, Nemoto T (2006) Rapidly growing dysembryoplastic neuroepithelial tumorcase report. Neurosurgery 59(6):E1337–E1338. https://doi.org/10.1227/01.NEU.0000245621.62721.79
Chao L, Tao XB, Jun YK, Xia HH, Wan WK, Tao QS (2013) Recurrence and histological evolution of dysembryoplastic neuroepithelial tumor: a case report and review of the literature. Oncol Lett 6(4):907–914
Nadi M, Ahmad T, Huang A, Hawkins C, Bouffet E, Kulkarni AV (2016) Atypical teratoid rhabdoid tumor diagnosis after partial resection of dysembryoplastic neuroepithelial tumor: case report and review of the literature. Pediatr Neurosurg 51(4):191–198
Ray WZ, Blackburn SL, Casavilca-Zambrano S, Barrionuevo C, Orrego JE, Heinicke H, Dowling JL, Perry A (2009) Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: a report of 5 cases and review of the literature. J Neurooncol 94(2):283. https://doi.org/10.1007/s11060-009-9849-9
Stokland T, Liu J-F, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro-Oncology 12(12):1257–1268. https://doi.org/10.1093/neuonc/noq092
Nolan M, Sakuta R, Chuang N, Otsubo H, Rutka J, Or Snead, Hawkins C, Weiss S (2004) Dysembryoplastic neuroepithelial tumors in childhood long-term outcome and prognostic features. Neurology 62(12):2270–2276
Chassoux F, Rodrigo S, Mellerio C, Landre E, Miquel C, Turak B, Laschet J, Meder JF, Roux FX, Daumas-Duport C, Devaux B (2012) Dysembryoplastic neuroepithelial tumors: an MRI-based scheme for epilepsy surgery. Neurology 79(16):1699–1707. https://doi.org/10.1212/WNL.0b013e31826e9aa9
Chang EF, Christie C, Sullivan JE, Garcia PA, Tihan T, Gupta N, Berger MS, Barbaro NM (2010) Seizure control outcomes after resection of dysembryoplastic neuroepithelial tumor in 50 patients. J Neurosurg Pediatr 5(1):123–130
Sakuta R, Otsubo H, Nolan MA, Weiss SK, Hawkins C, Rutka JT, Chuang NA, Chuang SH, Carter Snead O (2004) Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol 19(3):377–384
Qaddoumi I, Ellison DW, Morris EB, Broniscer A, Boop F, Merchant T, Palmer SL, Gajjar A (2010) Dysembryoplastic neuroepithelial tumors and cognitive outcome. Cancer 116(23):5461–5469. https://doi.org/10.1002/cncr.25528
Haydon DH, Dahiya S, Smyth MD, Limbrick DD, Leonard JR (2014) Greater extent of resection improves ganglioglioma recurrence-free survival in children: a volumetric analysis. Neurosurgery 75(1):37–42. https://doi.org/10.1227/neu.0000000000000349
Ranger A, Diosy D (2015) Seizures in children with dysembryoplastic neuroepithelial tumors of the brain—a review of surgical outcomes across several studies. Child’s Nervous System 31(6):847–855. https://doi.org/10.1007/s00381-015-2675-9
Bilginer B, Yalnızoglu D, Soylemezoglu F, Turanlı G, Cila A, Topçu M, Akalan N (2008) Surgery for epilepsy in children with dysembryoplastic neuroepithelial tumor: clinical spectrum, seizure outcome, neuroradiology, and pathology. Child’s Nervous System 25(4):485. https://doi.org/10.1007/s00381-008-0762-x
Francine C, Catherine D-D (2013) Dysembryoplastic neuroepithelial tumors: where are we now? Epilepsia 54(s9):129–134. https://doi.org/10.1111/epi.12457
Englot DJ, Berger MS, Barbaro NM, Chang EF (2012) Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia 53(1):51–57. https://doi.org/10.1111/j.1528-1167.2011.03269.x
Thom M, Toma A, An S, Martinian L, Hadjivassiliou G, Ratilal B, Dean A, McEvoy A, Sisodiya SM, Brandner S (2011) One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol 70(10):859–878. https://doi.org/10.1097/NEN.0b013e3182302475
Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R, Marliani AF, Bisulli F, Tinuper P, Castana L, Sartori I, Calbucci F (2009) Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg 111(6):1275–1282. https://doi.org/10.3171/2009.3.Jns081350
Nitin T, Yoshua E (2013) Resection strategies in tumoral epilepsy: is a lesionectomy enough? Epilepsia 54(s9):72–78. https://doi.org/10.1111/epi.12448
André P, Eliseu P, Duval SV (2013) Developmental tumors and adjacent cortical dysplasia: single or dual pathology? Epilepsia 54(s9):18–24. https://doi.org/10.1111/epi.12438
Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, Zeinieh M, Blanc R, Burk DL, Fahiminiya S, Bareke E, Schuller U, Monoranu CM, Strater R, Kerl K, Niederstadt T, Kurlemann G, Ellezam B, Michalak Z, Thom M, Lockhart PJ, Leventer RJ, Ohm M, MacGregor D, Jones D, Karamchandani J, Greenwood CM, Berghuis AM, Bens S, Siebert R, Zakrzewska M, Liberski PP, Zakrzewski K, Sisodiya SM, Paulus W, Albrecht S, Hasselblatt M, Jabado N, Foulkes WD, Majewski J (2016) Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131(6):847–863. https://doi.org/10.1007/s00401-016-1549-x
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131(6):833–845
Blümcke I, Aronica E, Becker A, Capper D, Coras R, Honavar M, Jacques TS, Kobow K, Miyata H, Mühlebner A (2016) Low-grade epilepsy-associated neuroepithelial tumours—the 2016 WHO classification. Nat Rev Neurol 12(12):732
Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A (2012) De novo somatic mutations in components of the PI3 K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 44(8):941
Lim JS, W-i Kim, Kang H-C, Kim SH, Park AH, Park EK, Cho Y-W, Kim S, Kim HM, Kim JA (2015) Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21(4):395
Acknowledgements
We thank our patients and their families for their kind contribution to the research.
Author information
Authors and Affiliations
Contributions
JY, SKK, KCW, JHP contributed to the design, data analysis, manuscript drafting and editing of the study. KJK, JHC, BCL, SHP contributed to acquisition and analysis of data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (Approval No. H-1807-067-958). Obtaining Informed consent was waived by the IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Kim, SK., Kim, K.J. et al. Satellite lesions of DNET: implications for seizure and tumor control after resection. J Neurooncol 143, 437–445 (2019). https://doi.org/10.1007/s11060-019-03174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03174-3