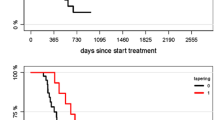
Abstract
Rechallenge with temozolomide has been shown to be a valid option in selected patients with progressive glioblastoma. Herein, we assessed the efficacy of rechallenge with bevacizumab in glioblastoma patients progressing off therapy. We retrospectively identified and analyzed the characteristics of patients with glioblastoma rechallenged with a bevacizumab-based chemotherapy regimen after having received bevacizumab as first-line treatment in association with temozolomide radiochemotherapy or at recurrence in association with temozolomide, CCNU or irinotecan. Twenty-five patients were identified. In all included patients, the first bevacizumab treatment resulted in an objective response and was discontinued for reasons other than disease progression (adverse event n = 9, physician or patient decision n = 16). Median duration of first bevacizumab treatment was 6 months (range: 2–58 months). None of the patients presented a rebound effect after bevacizumab discontinuation. The median interval between discontinuation of first bevacizumab treatment and bevacizumab rechallenge was 8.9 months (range: 2–58 months). At this time, bevacizumab was given in association with lomustine (n = 17), temozolomide (n = 6), irinotecan (n = 1), or alone (n = 1). Bevacizumab rechallenge resulted in an objective response in 15 patients (60%). Median progression-free survival was 6.7 months and overall survival was 9.6 months after bevacizumab rechallenge. Timing of first bevacizumab treatment (as first-line treatment or at recurrence) was not associated with the duration of response after treatment rechallenge. In the present series, patients who responded to bevacizumab and in whom this treatment was discontinued in the absence of tumor progression seemed to benefit from rechallenge with a bevacizumab-based chemotherapy regimen.


Similar content being viewed by others
References
Weller M, van den Bent M, Tonn JC et al (2017) European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18(6):e315–e329
Weller M, Tabatabai G, Kästner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res 21(9):2057–2064
Meyronet D, Esteban-Mader M, Bonnet C et al (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 9(8):1127–1134
Van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol 20(18):2905–2908
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Wick W, Gorlia T, Bendszus M et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Chinot OL, Nishikawa R, Mason W et al (2016) Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio. Neuro Oncol 18(9):1313–1318
Tabouret E, Boudouresque F, Farina P et al (2015) MMP2 and MMP9 as candidate biomarkers to monitor bevacizumab therapy in high-grade glioma. Neuro Oncol 17(8):1174–1176
Sandmann T, Bourgon R, Garcia J et al (2015) Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio Trial. J Clin Oncol 1(25):2735–2744
Erdem-Eraslan L, Van den Bent MJ, Hoogstrate Y (2016) Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB Trial. Cancer Res 76(3):525–534
Dehais C, Souvannavong V, Nguyen BK (2011) An evaluation of fatigue in patients with glioblastoma relapse treated with the combination of irinotecan-bevacizumab. Rev Neurol (Paris) 167(11):841–846
Zuniga RM, Torcuator R, Jain R et al (2010) Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol 99(2):237–242
Hertenstein A, Hielscher T, Menn O et al (2016) Impact of tapering and discontinuation of bevacizumab in patients with progressive glioblastoma. J Neurooncol 129(3):533–539
Cha Y, Kim YJ, Lee SH et al (2017) Post-bevacizumab clinical outcomes and the impact of early discontinuation of bevacizumab in patients with recurrent malignant glioma. Cancer Res Treat 49(1):129–140
Anderson MD, Hamza MA, Hess KR, Puduvalli VK (2014) Implications of bevacizumab discontinuation in adults with recurrent glioblastoma. Neuro Oncol 16(6):823–828
Reardon DA, Galanis E, DeGroot JF (2011) Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol 13(3):353–361
Schreuer M, Jansen Y, Planken S et al (2017) Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol 18(4):464–472
Cappuzzo F, Morabito A, Normanno N et al (2016) Efficacy and safety of rechallenge treatment with gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer 99:31–37
Oudard S, Geoffrois L, Guillot A et al (2016) Clinical activity of sunitinib rechallenge in metastatic renal cell carcinoma-Results of the REchallenge with SUnitinib in MEtastatic RCC (RESUME) Study. Eur J Cancer 62:28–35
Kuczynski E, Sargent D, Grothey A, Kerbel R (2013) Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat Rev Clin Oncol 10(10):571–587
Perry JR, Bélanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28(12):2051–2057
Reardon DA, Herndon JE, Peters KB et al (2012) Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer 107(9):1481–1487
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Rahman R, Hempfling K, Norden AD et al (2014) Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab. Neuro Oncol 16(11):1523–1529
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Bronnimann, C., Izquierdo, C., Cartalat, S. et al. Rechallenge with bevacizumab in patients with glioblastoma progressing off therapy. J Neurooncol 138, 141–145 (2018). https://doi.org/10.1007/s11060-018-2780-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2780-1